Advanced Biological Laboratories (ABL) S.A Inks Licensing Agreement with the Institut Pasteur for HPV RNA-Seq Technology
HPV Transcriptome as a Game-Changing Biomarker for Detection, Genotyping, and Monitoring of HPV-Associated Cervical High-Grade Cytology
LUXEMBOURG, LUXEMBOURG, April 22, 2025 /EINPresswire.com/ — Advanced Biological Laboratories (ABL), a diagnostics company, headquartered in Luxembourg, is pleased to announce a licensing agreement with the renowned Institut Pasteur for the integration of the innovative HPV RNA-Seq technology into its diagnostic solutions.
HPV RNA-Seq is a cutting-edge diagnostic method that enhances the detection and typing of high-risk human papillomavirus (HPV) infections. This technology not only identifies the presence of HPV but also determines the specific viral strains and assesses the risk of progression to cervical cancer. HPV RNA-Seq demonstrated superior sensitivity compared to traditional DNA-based tests, detecting additional HPV-positive cases and multiple infections that were previously unrecognized (https://doi.org/10.1016/j.jmoldx.2019.04.010).
Furthermore, HPV RNA-Seq technology is already capable of competing with cytology tests, providing a highly sensitive and precise alternative for HPV monitoring. Its advanced capabilities make it a true game changer, as it has the potential to ultimately replace HPV screening tests altogether. By offering a more reliable method for early detection and risk assessment, this technology revolutionizes current strategies for monitoring HPV patients and significantly enhances patient care.
By incorporating HPV RNA-Seq into its product portfolio, ABL aims to provide healthcare professionals with more precise and comprehensive tools for early detection and management of HPV-related conditions. This advancement aligns with the company’s commitment to enhancing patient outcomes through innovative diagnostic solutions.
“We are excited to collaborate with the Institut Pasteur to bring this state-of-the-art technology to clinicians worldwide,” said Dr. Chalom Sayada, CEO of ABL. “This agreement underscores our dedication to advancing molecular diagnostics and improving the accuracy of HPV screening. HPV RNA-Seq is a disruptive innovation that is set to redefine how HPV infections are diagnosed and managed.”
“We worked for several years to develop the HPV RNA seq technology at the Institut Pasteur, together with Prof. Marc Eloit, inventor of the technology. Support from the Institut Pasteur Innovation Accelerator and guidance from the Technology Transfer and Industrial Partnership Department (DARRI) were decisive in achieving a sufficient TRL and envisaging an industrial transfer. The signature of the license with ABL marks an important milestone in the project. We are convinced that this collaboration will enable us to put this innovation at the service of clinicians and patients, thus contributing to the prevention of cervical cancer”, added Philippe Pérot, co-inventor of the HPV RNA-Seq technology and Expert Research Engineer at the Institut Pasteur.
The global market for HPV testing and related disease monitoring is experiencing significant growth, driven by the increasing prevalence of cervical cancer and heightened awareness of early detection methods. In 2022, the market was valued at approximately USD 3.90 billion and is projected to grow at a compound annual growth rate (CAGR) of 11.8%, reaching an estimated USD 9.33 billion by 2030. (GrandViewResearch, Report ID: 978-1-68038-895-4).
This robust growth underscores the escalating demand for advanced diagnostic tools in HPV detection and genotyping. With the introduction of HPV RNA-Seq, ABL is at the forefront of a transformation in HPV diagnostics, providing a superior alternative to traditional screening methods and paving the way for more effective disease prevention strategies.
The financial terms of the agreement remain undisclosed.
***
Full Press Release
ABL Inks Know-How License and Transfer Agreement for the Fast Track Diagnostics PCR Portfolio from Siemens Healthineers
ABL and affiliates plan to start the production and commercialization of new UltraGene products based on PCR tests previously supplied by Fast Track Diagnostics
ABL Diagnostics SA (EPA:ABLD)
LUXEMBOURG VILLE, LUXEMBOURG, February 3, 2025 /EINPresswire.com/ — Advanced Biological Laboratories (ABL), a diagnostics company, headquartered in Luxembourg, announces that the licensing and transfer agreement of know-how and IP rights from Fast Track Diagnostics Luxembourg (FTD) has been inked with Siemens Healthineers.
FTD was engaged in the development, manufacturing and selling of in-vitro diagnostic and research use only molecular testing products. Siemens Healthineers, as the owner of FTD, licensed and transferred the know-how and IP rights to ABL and its affiliates (ABL Group) related to the design and manufacturing of certain former FTD products.
Based on this Know-How License and Transfer Agreement (Agreement) the ABL group intends to manufacture and commercialize its own testing products equivalent to the former FTD products. Financial details of the deal were not disclosed.
Full Press Release
Webinar: E-AHPBA–ESSO–ESSR Innsbruck consensus guidelines for preoperative liver function assessment before hepatectomy
22.11.2023 at 4:30 to 6:00 p.m. (CET)
These European guidelines for the preoperative measurement of liver function were published in June of this year.
This represents an important milestone for the use of the LiMAx test and underlines its importance for the safety of patients and surgeons.
In our webinar, we want to highlight the key aspects of these guidelines, including the resulting significance for the use of the LiMAx test in daily practice. In addition, we will discuss applications of the LiMAx test in hepatology.
program:
Chairman: Martin Stockmann
Johan Lock: Key points of the E-AHPBA-ESSO-ESSR guidelines
Martin Stockmann: The LiMAx test in the E-AHPBA-ESSO-ESSR guidelines and recommendations for day-to-day practice
Guido Gerken: The use of the LiMAx test in hepatology: study data, personal experience and billing tips
You can attend the webinar on the announced date by using the following link:
Join LiMAx Webinar (EU Consensus Guideline)
LiMAx® Functional Principle:
LiMAx (Liver Maximum Capacity) is a diagnostic breath test for the quantitative determination of the maximum enzymatic liver function in adults. The test is based on the metabolization of a non-radioactive and isotope labeled drug, 13C-Methacetin by the cytochrome-P450 (CYP1A2) enzyme. Due to this metabolization the 13CO2 levels in the exhaled air increases. The increase is a direct measure of liver function capacity at the cellular level, called LiMAx.
For further information: www.humedics.eu
Contact for journalists & editors:
Dr. Ralf Kohnen
Humedics GmbH
Bundesallee 23
10717 Berlin
Tel.: +49 (0) 30 6293955 0
Fax: +49 (0) 30 6293955 30
info@humedics.de
www.humedics.eu
Press release distributed by Pressat on behalf of Humedics GmbH, on Monday 13 November, 2023. For more information subscribe and follow https://pressat.co.uk/
CytoSorbents And Humedics Announce Theranostic Collaboration in the Field of Liver Disease
PRINCETON, N.J., July 11, 2023 (GLOBE NEWSWIRE) — CytoSorbents Corporation (NASDAQ: CTSO), a leader in the treatment of life-threatening conditions in the intensive care unit and cardiac surgery using blood purification via its proprietary polymer adsorption technology, and Humedics GmbH, a leader in non-invasive liver function measurement, have announced a theranostic collaboration in the field of liver disease.
- CytoSorbents’ CytoSorb® blood purification technology is expanding the dimension of blood purification as the only E.U. approved extracorporeal liver support therapy specifically approved to remove both bilirubin, a key liver toxin that accumulates during liver dysfunction and failure, and cytokines, where cytokine storm is often the trigger or exacerbating factor in acute and acute-on-chronic liver disease. CytoSorb is easy and quick to setup and use, and removes other liver toxins such as bile acids and a wide range of inflammatory mediators. CytoSorb has been used for this application and many others in more than 200,000 human treatments in 75 countries around the world to date.
- Humedics is the pioneer of LiMAx®, a rapid and precise E.U. approved diagnostic using its innovative breath analysis technology to quantitatively assess liver function. This easy-to-use bedside test provides doctors with accurate information to both monitor liver function in compromised patients longitudinally, and to support clinical decisions, such as the need to initiate extracorporeal liver support in critically ill patients, or the extent of liver resection in cancer patients with liver involvement. LiMAx has already been used on thousands of patients in Europe.
CytoSorbents and Humedics will collaborate on a joint product promotion and comarketing program aimed at increasing market awareness, adoption, usage, and sales of their respective technologies in the liver field. A key goal is to generate new customer leads for both CytoSorb and LiMAx through cross-training and selling amongst the companies’ combined sales teams at relevant accounts. The program will commence immediately starting in Germany, the United Kingdom, France, Austria, Switzerland, Belgium, Netherlands, Luxembourg, Finland, Norway, Sweden, and Poland. CytoSorbents and Humedics also plan to work together to bring new innovative solutions to the market which may include future collaboration opportunities in clinical studies, new therapeutic areas and indications, and new product concepts.
“Acute and chronic liver disease is a massive and growing problem throughout the world, afflicting 1 in every 11 people,” said Dr. Christian Steiner, Executive Vice President of Sales and Marketing of CytoSorbents. “These illnesses are often associated with untreated excessive levels of cytokines, bilirubin, and other liver toxins that can worsen liver and other organ dysfunction. Today, there are limited treatment options available that are typically not utilized due to poor or inadequate efficacy, usability, and high costs. We believe CytoSorb addresses these issues, which represents a significant potential market opportunity for us. We are pleased to team with Humedics to develop a much more impactful personalized medicine strategy to diagnose, monitor, and treat liver disease with our combined theranostics approach.”
Dr. Ralf Kohnen, Chief Business Officer of Humedics, commented, “Our proprietary LiMAx breath analysis technology provides liver surgeons, hepatologists and other liver specialists the ability to easily assess liver function capacity. LiMAx produces a quantitative measure of maximal liver function, revealing liver metabolic function at the cellular level in real time, which correlates with liver disease severity. This information can be used by clinicians to monitor the efficacy of different therapies, predict prognoses, and to plan hepatic resections in liver cancer to ensure that the remaining liver has sufficient function. We view this collaboration with CytoSorbents as a natural evolution of our collective approaches and look forward to the synergy we can create in the marketplace.”
“This program has the potential to benefit both companies by enabling them to accelerate the introduction of innovative new diagnostic and therapeutic product offerings for hepatologists and liver support specialists that are dealing with the millions of patients suffering from liver disease each day,” stated Chris Cramer, Senior Vice President of Business Development for CytoSorbents. “This is a great opportunity to rapidly bring the benefits of LiMAx and our CytoSorb therapy to whole new segment of customers that may not yet know about these products.”
The financial terms of this agreement have not been disclosed.
About Humedics GmbH
Humedics, based in Berlin, focuses on rapid and precise liver function measurement using LiMAx, its innovative breath analysis technology. This easy-to-use bedside test provides doctors with accurate information to support decision making in tailoring the medical treatment to the individual level of each patient and to improve patient outcomes. LiMAx has already been used on thousands of patients and the company is currently expanding its operation globally.
About CytoSorbents Corporation (NASDAQ: CTSO)
CytoSorbents Corporation is a leader in the treatment of life-threatening conditions in the intensive care unit and in cardiac surgery through blood purification. Its lead product, CytoSorb®, is approved in the European Union and distributed in 75 countries worldwide. It is an extracorporeal cytokine adsorber that reduces “cytokine storm” or “cytokine release syndrome” in common critical illnesses that can lead to massive inflammation, organ failure and patient death. In these diseases, the risk of death can be extremely high, and there are few, if any, effective treatments. CytoSorb is also used during and after cardiothoracic surgery to remove antithrombotic drugs and inflammatory mediators that can lead to postoperative complications, including severe bleeding and multiple organ failure. At the end of Q1 2023, more than 203,000 CytoSorb devices had been used cumulatively. CytoSorb was originally launched in the European Union under CE mark as the first cytokine adsorber. Additional CE mark extensions were granted for bilirubin and myoglobin removal in clinical conditions such as liver disease and trauma, respectively, and for ticagrelor and rivaroxaban removal in cardiothoracic surgery procedures. CytoSorb has also received FDA Emergency Use Authorization in the United States for use in adult critically ill COVID-19 patients with impending or confirmed respiratory failure. The DrugSorb™-ATR antithrombotic removal system, based on the same polymer technology as CytoSorb, also received two FDA Breakthrough Device Designations, one for the removal of ticagrelor and another for the removal of the direct oral anticoagulants (DOAC) apixaban and rivaroxaban in a cardiopulmonary bypass circuit during urgent cardiothoracic procedures. The Company is currently conducting the FDA-approved, randomized, controlled STAR-T (Safe and Timely Antithrombotic Removal-Ticagrelor) study of 120 patients at approximately 30 centers in U.S. and Canada to evaluate whether intraoperative use of DrugSorb-ATR can reduce the perioperative risk of bleeding in patients receiving ticagrelor and undergoing cardiothoracic surgery. This pivotal study is intended to support U.S. FDA and Health Canada marketing approval for DrugSorb-ATR in this application.
CytoSorbents’ purification technologies are based on biocompatible, highly porous polymer beads that can actively remove toxic substances from blood and other bodily fluids by pore capture and surface adsorption. Its technologies have received non-dilutive grant, contract, and other funding of approximately $48 million from DARPA, the U.S. Department of Health and Human Services (HHS), the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI), the U.S. Army, the U.S. Air Force, U.S. Special Operations Command (SOCOM), Air Force Material Command (USAF/AFMC), and others. The Company has numerous marketed products and products under development based upon this unique blood purification technology protected by many issued U.S. and international patents and registered trademarks, and multiple patent applications pending, including ECOS-300CY®, CytoSorb-XL™, HemoDefend-RBC™, HemoDefend-BGA™, VetResQ®, K+ontrol™, DrugSorb™, DrugSorb™-ATR, ContrastSorb, and others. For more information, please visit the Company’s websites at www.cytosorbents.com and www.cytosorb.com or follow us on Facebook and Twitter.
Forward-Looking Statements
This press release includes forward-looking statements intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, statements about our plans, objectives, future targets and outlooks for our business, statements about potential exposures resulting from our cash positions, representations and contentions, and are not historical facts and typically are identified by use of terms such as “may,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue” and similar words, although some forward-looking statements are expressed differently. You should be aware that the forward-looking statements in this press release represent management’s current judgment and expectations, but our actual results, events and performance could differ materially from those in the forward-looking statements. Factors which could cause or contribute to such differences include, but are not limited to, the risks discussed in our Annual Report on Form 10-K, filed with the SEC on March 9, 2023, as updated by the risks reported in our Quarterly Reports on Form 10-Q, and in the press releases and other communications to shareholders issued by us from time to time which attempt to advise interested parties of the risks and factors which may affect our business. We caution you not to place undue reliance upon any such forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, other than as required under the Federal securities laws.
Please Click to Follow Us on Facebook and Twitter
Contacts Humedics GmbH
Bundesallee 23
10717 Berlin, Germany
Phone +49 30 629 39 55-0
Fax +49 30 629 39 55-30
info@humedics.de
CytoSorbents Corporation Contact:
Kathleen Bloch, Interim CFO
(732) 398-5429
kbloch@cytosorbents.com
CytoSorbents Europe GmbH:
Josephine Kraus
PA by Dr. Christian Steiner
+49 30 765 84 66 23
josephine.kraus@cytosorbents.com
ABL SA group acquires Berlin based Medtech company Humedics
ABL SA acquires the company Humedics Gmbh to extend the use of a robust, well-known, and CE-marked technology intended to be used for liver function measurement. Financial terms are not disclosed.
Doctor Chalom Sayada, founder of the ABL SA and managing director of the Humedics declared: “We are proud to take over Humedics, and help promoting and expanding such a technology at the international level.”
A new dedicated strategy is planned for supporting the growth of the Berlin-based company in the future and to establish the LiMAx test as the gold standard for liver function measurement worldwide. In cooperation with well-known scientific institutions, the Humedics technology, which was originally developed for use in liver surgery, is to be expanded to the entire hepatological area, thereby avoiding invasive medical interventions in the future. Finally, remarked Dr. Chalom Sayada: “We are convinced that by acquiring the technology and the know-how of the employees, we will create added value for the benefit of patients.”
Further information at:
www.ablsa.com
www.humedics.eu
About Humedics
Based in Berlin, Germany, Humedics GmbH is specialised in rapid and accurate liver function measurement using the LiMAx® test, an innovative technology for analysing exhaled air. The LiMAx® test provides physicians with a method for quantitatively assessing an individual patient’s liver function capacity within minutes. This enables physicians to select treatment strategies and follow disease progression based on up-to-date knowledge of the functional health of the liver.
Current applications, which have already been published in prestigious medical journals, include liver function diagnosis before and after liver transplantation, surgical planning for liver surgery (such as assessing how much liver tissue can be removed without increasing the risk of acute liver failure), and assessment of liver diseases such as fibrosis and cirrhosis.
At present, more than 20 studies regarding new diagnostic indications are being conducted. These include diagnosing and staging of chronic liver diseases such as non-alcoholic steatohepatitis (NASH), as well as selecting, monitoring and managing different oncology treatment options. These studies, initiated by researchers, have already provided evidence of further potential for the LiMAx® tests.
The LiMAx® test is in routine clinical use at more than 30 leading university hospitals in Europe. So far, more than 20,000 LiMAx® tests have been performed. The LiMAx® test is commercially available in Germany, Austria and the UK.
Advanced Biological Laboratories Fedialis SAS (formerly ABL France SAS) is now ABL Diagnostics SA
On September 1st 2022, Advanced Biological Laboratories Fedialis SAS (formerly ABL France SAS) merged into ABL Diagnostics SA (ABLD), a worldwide leading international company, listed on Euronext Paris compartment C (ISIN FR001400AHX6), offering innovative and proprietary molecular biology assays and end-to-end solutions intended to be used for molecular detection by Polymerase Chain Reaction (PCR) – UltraGene® and for genotyping through DNA sequencing – DeepChek® (a very sensitive, robust and sustainable technology allowing precise identification of relevant genomic variations like single nucleotide polymorphisms (SNP), amino-acid mutations, quasispecies like variants of concern, already published or which will be discovered in the future, with known impact on disease prognosis, drug efficacy, pathogen activity…).
These molecular biology products are generating recurring revenues and cover one of the largest portfolio of microbiology applications, growing fast year after year to stick to the market needs, with a primary focus on HIV (with CE-IVD marked target-specific assays covering all relevant genes used for drug resistance assessment like reverse transcriptase, protease, integrase and with disruptive Whole Genome Kits), on SARS-CoV-2 (with a CE-IVD marked Whole Genome assay), on Tuberculosis (with a CE-IVD marked multiplex assay targeting genes relevant for first line, second line and new-drugs resistance determination), on viral hepatitis B and C, 16s/18s RNA for taxonomy and microbiome analyses and other viral and bacterial targets. Please consult ABL team for further information about registration status of the ABLD’s products in your territory.
ABLD commercializes its entire line of products on a worldwide basis through its own sales team and through a network of exclusive distributors active on all continents. ABLD clients are academic clinical pathology labs, private reference labs and researchers willing to implement an innovative and robust microbiology content in constant expansion.
ABLD also develops, manufactures and markets kits for clinical specimen collection – MediaChek® and digital solutions like Nadis®, an CE-marked Electronic Medical Record (EMR) system used in France in more than 200 hospitals managing patients infected by HIV or Viral Hepatitis.
Since 2019, the activities of ABL DIAGNOSTICS have also been carried out in the United States through its wholly owned subsidiary, ABL ADVANCEDDX BIOLOGICAL LABORATORIES USA Inc.
For more information, visit www.abldiagnostics.com.
ABL DIAGNOSTICS S.A.
42, rue Olivier Métra – Bat E1
75020, PARIS FRANCE
Phone : +33 145 061 574
Email : contact@abldiagnostics.com
ABL SA
52-54 avenue du X septembre
L-2550
LUXEMBOURG
Email : contact@ablsa.com
REALISATION DE LA FUSION
Paris, le 31 août 2022,
Lors de la réunion du 3 août 2022 et dans le prolongement des communications effectuées à ce sujet, l’assemblée générale des actionnaires de la société ABL Diagnostics (la « Société ») a approuvé le projet de fusion-absorption de la société Advanced Biological Laboratories Fedialis SAS par la Société (la « Fusion »), les apports réalisés au titre de la Fusion et la rémunération desdits apports conformément aux termes du traité de fusion conclu le 14 juin 2022.
Les informations concernant la société absorbée et les modalités de la Fusion figurent dans le prospectus établi par la Société conformément aux dispositions du Règlement (UE) 2017/1129 visé par l’AMF le 12 juillet 2022 sous le numéro de visa 22-296.
Il est rappelé, ainsi que cela a été indiqué dans les communications précédentes de la Société, que la Fusion serait précédée d’une opération technique de réduction de capital par voie de la réduction de la valeur nominale unitaire de 1 euro à 0,10 euro (la « Réduction de Capital »).
Le Conseil d’administration de la Société réuni ce jour a constaté la réalisation de l’ensemble des conditions suspensives préalables à la Fusion, consistant en (i) la remise des rapports des commissaires à la fusion sur la valeur et la rémunération des apports conformément à la réglementation applicable, (ii) la décision de l’AMF du 19 juillet 2022 constatant qu’il n’y avait pas lieu au dépôt d’une offre publique de retrait préalable sur le fondement de l’article 236-6 du règlement général, (iii) l’approbation du prospectus (susvisé) par l’AMF, (iv) l’approbation par l’assemblée générale de la Société (susvisée) de la Réduction de Capital préalable, de la Fusion et de l’augmentation de capital en résultant, (v) la réalisation de la Réduction de Capital et (vi) l’approbation de la Fusion par l’associé unique de la société Advanced Biological Laboratories Fedialis SAS.
Le Conseil d’administration a ainsi constaté ce jour la réalisation définitive de la Fusion et de ses conséquences notamment la réalisation de l’augmentation de capital et la dissolution sans liquidation de la société absorbée.
Par l’effet de la Fusion, conformément aux décisions de l’assemblée générale du 3 août 2022, le capital social de la Société est porté de 200.648 euros (tel que résultant de la Réduction de Capital réalisée ce jour) à 1.611.465,60 euros par l’émission de 14.108.176 actions nouvelles de 0,10 euro de valeur nominale chacune entièrement libérées et attribuées à la société Advanced Biological Laboratories SA, associé unique de la société absorbée. Les actions nouvelles résultant de la Fusion seront admises sur Euronext Paris à partir du 2 septembre 2022.
Il est rappelé que si la Fusion est juridiquement réalisée ce jour, la date d’effet comptable et fiscale a été fixée au 1er janvier 2022.
A propos d’ABL Diagnostics
ABL Diagnostics est cotée sur Euronext Paris, compartiment C (code ISIN FR001400AHX6).
Plus d’informations sur https://www.abldiagnostics.com/
(ou sur www.fauvet-girel.fr qui reste encore actif à titre transitoire)
Contacts
ABL Diagnostics
+33 (0)1 56 88 20 40
fauvet-girel@ablsa.com
WHO algorithm v2021-06 for Tuberculosis resistance now available!
We are pleased to announce the immediate availability of the WHO Catalog of Mutations (version 2021-06) in BacterioChek-TB, intended to be used for Mycobacterium tuberculosis drug resistance assessement.
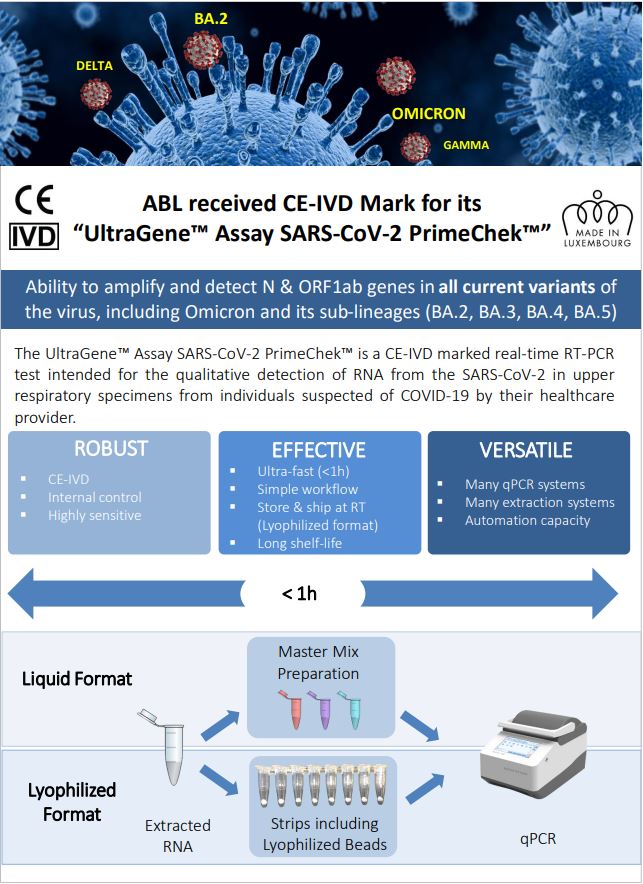
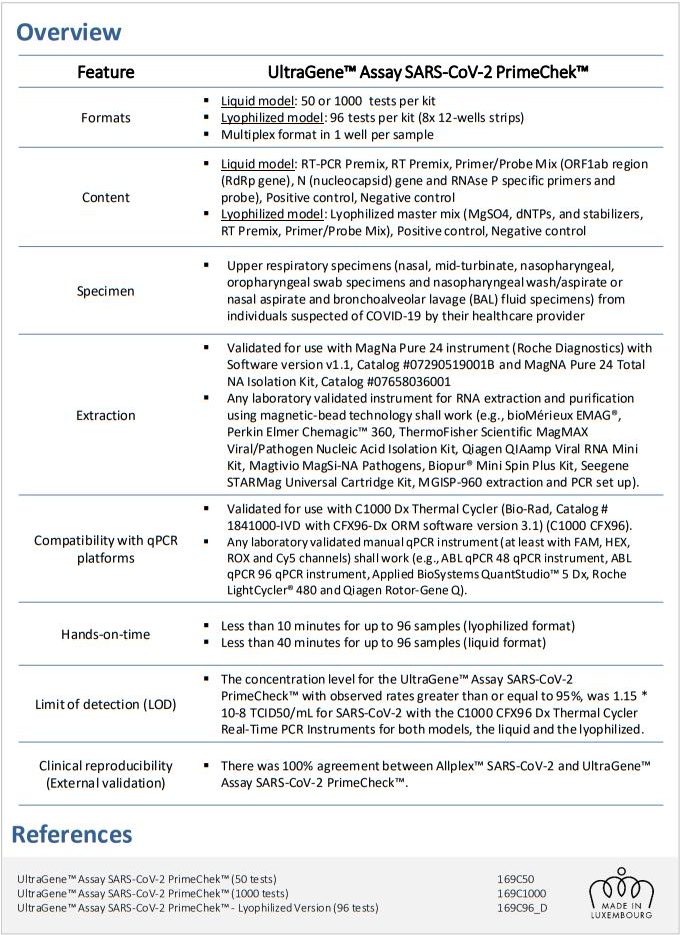
ABL received CE-IVD Mark for its “UltraGene™ Assay SARS-CoV-2 PrimeChek™”
The UltraGene™ Assay SARS-CoV-2 PrimeChek™ is a CE-IVD marked real-time RT-PCR test intended for the qualitative detection of RNA from the SARS-CoV-2 in upper respiratory specimens from individuals suspected of COVID-19 by their healthcare provider.
New Distribution Partners in Georgia & Paraguay !
This week we are proud to have two new Distribution Partners on our side :
– ELTA 90 Medical Genetics in Georgia
– Infotec in Paraguay
Both agreements will cover all ABL’s Diagnostics line of products, including the DeepChek® Assays and Softwares.

SIGNATURE D’UN TRAITE DE FUSION
Le Conseil d’administration de la société ABL Diagnostics (la « Société »), réuni le 13 juin 2022, a approuvé le projet de fusion-absorption aux termes duquel il est prévu que la Société absorbe la société Advanced Biological Laboratories Fedialis (« ABL France »), chacune des deux sociétés étant contrôlées par la société Advanced Biological Laboratories SA (« ABL SA »).
Dans ce cadre, la Société et ABL France ont signé le 14 juin 2022 un traité de fusion arrêtant les modalités économiques, financières et juridiques de la fusion.
Motifs et modalités de la fusion
Ce projet de fusion fait suite à l’acquisition par ABL SA, le 15 octobre 2021, de blocs d’actions lui ayant conféré 96,70% du capital et des droits de vote de la Société. Cette acquisition a donné lieu au dépôt par ABL SA d’un projet d’offre publique d’achat simplifiée sur les actions de la Société le 10 novembre 2021 (visa AMF n°21-535, décision de conformité du 21 décembre 2021). A l’issue de l’offre qui s’est déroulée du 23 décembre 2021 au 7 janvier 2022, ABL SA détenait 97,08% du capital et des droits de vote de la Société.
Comme annoncé par la Société dans ses communications précédentes, ABL SA souhaite transférer l’activité de la société Advanced Biological Laboratories Fedialis (« ABL France ») à la Société et ainsi réorienter l’activité de cette dernière. L’objectif annoncé reste que la Société devienne le véhicule coté du groupe ABL, notamment en vue de permettre à la Société de faire appel au marché le cas échéant pour financer ses futurs investissements et d’accélérer le développement de ses activités dans le domaine du diagnostic par génotypage de maladies infectieuse.
Dans ce cadre, Messieurs Laurent Halfon et Antoine Legoux ont été désignés en qualité de commissaires à la fusion par ordonnance du Président du Tribunal de commerce de Paris du 6 décembre 2021. Ils remettront leurs rapports sur la valeur des apports et sur la rémunération de la fusion conformément à la réglementation applicable.
Préalablement à la fusion, et afin de permettre la libération des apports de la fusion, les actionnaires seront appelés à se prononcer sur une réduction du capital social de la Société d’un montant de 1.805.832 euros par réduction du nominal des actions de 1 euro à 0,10 euro ramenant ainsi le capital social de 2.006.480 euros à 200.648 euros.
La parité d’échange retenue dans le cadre de la fusion, elle-même basée sur les comptes sociaux des deux sociétés au 31 décembre 2021, est de soixante-sept (67) actions de la Société pour une (1) action d’ABL France. La parité d’échange susvisée a été déterminée sur la base des valorisations respectives des deux sociétés, fixée à 3.987.879 euros pour ABL Diagnostics (par référence au prix de l’offre publique clôturée le 7 janvier 2022) et à 28.040.000 euros pour ABL France, sur la base d’une valorisation multicritères.
Les actions nouvelles émises par la Société en rémunération des apports de la fusion seront admises aux négociations sur Euronext Paris.
Les actionnaires de la Société seront appelés à se réunir en Assemblée Générale Mixte afin d’approuver la réduction de capital et la fusion, ainsi que la modification de l’objet social de la Société en résultant.
A l’issue de ces opérations, compte-tenu des valorisations respectives et de l’absence d’activité de l’absorbante, la Société sera toujours contrôlée très majoritairement par ABL SA.
Dans ce cadre, ABL SA déposera par ailleurs auprès de l’Autorité des marchés financiers (« AMF ») une demande aux fins de constater, conformément aux dispositions de l’article 236-6 de son règlement général, qu’il n’y a pas lieu au dépôt d’une offre publique de retrait.
Conditions suspensives de la réalisation de la fusion
La réalisation de la fusion est soumise aux conditions suspensives suivantes :
- la remise par les commissaires à la fusion (i) d’un rapport sur la valeur des apports et (ii) d’un rapport sur les conditions de la fusion ;
- la décision de l’AMF constatant qu’il n’y a pas lieu au dépôt d’une offre publique de retrait en application de l’article 236-6 du règlement général de l’AMF, purgée de tout recours ;
- l’approbation du prospectus relatif à la fusion par l’AMF ;
- l’approbation par l’assemblée générale des actionnaires de la Société de (i) la réduction de capital, (ii) de la fusion et (iii) de l’augmentation de capital en rémunération des apports au titre de la fusion ;
- la réalisation de la réduction de capital ; et
- l’approbation par l’associé unique d’ABL France (i) de la fusion et (ii) de la dissolution d’ABL France.
Le calendrier indicatif des opérations figure ci-dessous.
Calendrier indicatif (x)
| Dates |
Principales étapes |
| 14 juin 2022 |
– Signature du traité de fusion |
| 19 juillet 2022 |
– Décision de l’AMF de non-lieu au dépôt d’une offre publique de retrait en application de l’article 236-6 du règlement général de l’AMF |
| 28 juillet 2022 |
– Assemblée Générale Mixte de la Société approuvant (i) la réduction de capital et (ii) la fusion
– Assemblée Générale Extraordinaire d’ABL France approuvant la fusion |
| fin août 2022 |
– Fin du délai d’opposition de créanciers à la réduction de capital
– Réalisation de la réduction de capital
– Réalisation de la fusion |
(x) Dates envisagées
A propos d’ABL Diagnostics
ABL Diagnostics est cotée sur Euronext Paris, compartiment C (code ISIN FR001400AHX6).
Plus d’informations sur www.fauvet-girel.fr.
Contacts
ABL Diagnostics
+33 (0)1 56 88 20 40
fauvet-girel@ablsa.com
New Distribution Partnership in Ethiopia & Bolivia
We are pleased to announce that we entered into an exclusive distribution agreement 🤝 of ABL’s Diagnostics line of products, including the DeepChek® Assays 🔬and software:
– With Retina Pharmaceuticals in Ethiopia;
– With Bionova in Bolivia !

DeepChek® Assay 13-Plex KB Drug Susceptibility Testing is now CE-IVD marked
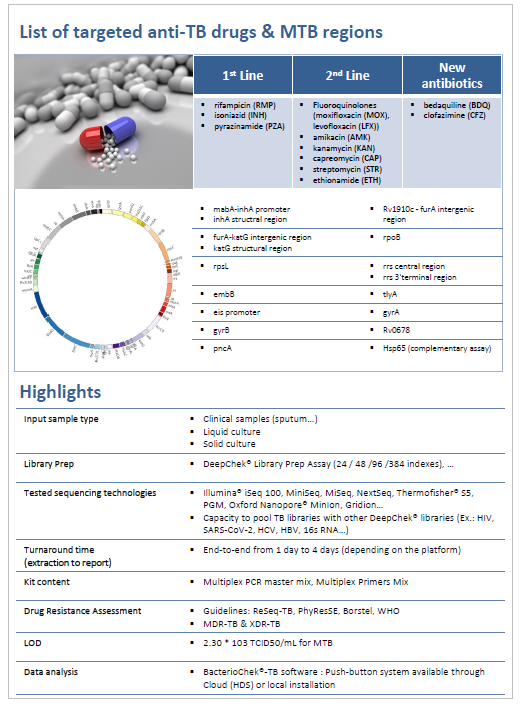
We are pleased to announce that our DeepChek® Assay 13-Plex KB Drug Susceptibility Testing, intended to be used for Tuberculosis Drug Resistance assessment through NGS sequencing, is now CE-IVD marked !


Presentation of the DeepChek-HIV Whole Genome Assay @ECCMID 2022
We are pleased to announce a new poster presentation entitled “Evaluation of a commercial assay whole genome HIV-1 using next-generation sequencing” at ECCMID 2022.
Link to the poster presentation.
Please feel free to contact us to get more information.
ETABLISSEMENTS FAUVET GIREL – RESULTATS ANNUELS 2021
ETABLISSEMENTS FAUVET GIREL (FR0000063034 – FAUV – Euronext Paris) publie ses résultats annuels au titre de l’exercice clos le 31 décembre 2021, arrêtés par le Conseil d’administration du 8 février 2022. Les comptes ont été audités par le commissaire aux comptes dont le rapport de certification est en cours d’émission.
Résultats annuels 2021
ETABLISSEMENTS FAUVET GIREL n’a plus d’activité opérationnelle depuis la cession de son activité de location de conteneurs et de wagons pour le fret ferroviaire en 2018 et ne détient plus aucun actif depuis la cession de son dernier actif immobilier situé à Meudon en avril 2021.
Les comptes sociaux de l’exercice écoulé font apparaitre un résultat net de 257 868 €, intégrant un résultat exceptionnel de 398 568 € lié à la cession de son dernier actif immobilier, contre une perte de (89 296) € pour l’exercice précédent.
Le total du bilan s’élève à 2 713 363 € contre 2 379 334 € pour l’exercice précédent.
L’endettement financier de la Société n’est pas significatif (intérêts courus à payer). La trésorerie d’exploitation d’un montant de 2 702 442 € au 31 décembre 2021 permet d’acquitter les dettes de la Société à échéance.
Perspectives – Opération de fusion-absorption au cours du 1er semestre 2022
Comme annoncé par la société Advanced Biological Laboratories SA lors de l’acquisition d’un bloc de contrôle de la Société, puis dans le cadre de l’offre publique d’achat simplifiée visant les actions de la Société, le projet de fusion-absorption de la société Advanced Biological Laboratories Fedialis (« ABL France ») par Etablissements Fauvet Girel est en cours de préparation, en vue d’une réalisation d’ici la fin du 1er semestre 2022.
Dans ce cadre, les actionnaires de la Société seront appelés à approuver en assemblée générale la fusion-absorption. L’admission des actions qui résulteront de cette opération sera conditionnée à l’approbation d’un prospectus par l’Autorité des marchés financiers.
A propos d’ETABLISSEMENTS FAUVET GIREL
ETABLISSEMENTS FAUVET GIREL est cotée sur Euronext Paris, compartiment C (ISIN FR0000063034).
Plus d’informations sur www.fauvet-girel.com.
Contacts
ETABLISSEMENTS FAUVET-GIREL
+33 (0)1 56 88 20 40
fauvet-girel@ablsa.com







