Two New Distributors
We are pleased to announce that we entered into an exclusive distribution agreement of ABL’s Diagnostics line of products, including the DeepChek® Assays and software:
With Integrated Medical Group LLC in Ukraine; and La Sénégalaise des Systèmes Médicaux (SSM) S.A in Senegal.
See current channel partners.
A new “Door To Door” service provided by ABL company !
The new gold standard for the clinical management of patients and of vaccinees in the context of viral evolution and the outgrowth of different Variants Of Concerns.

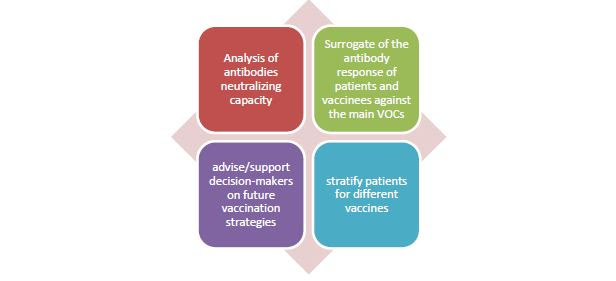
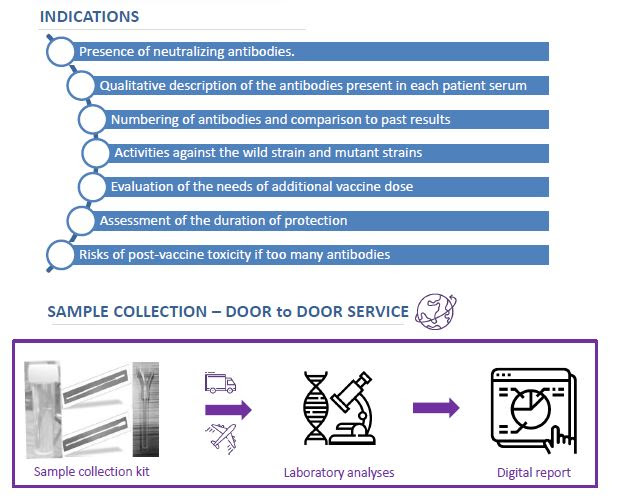

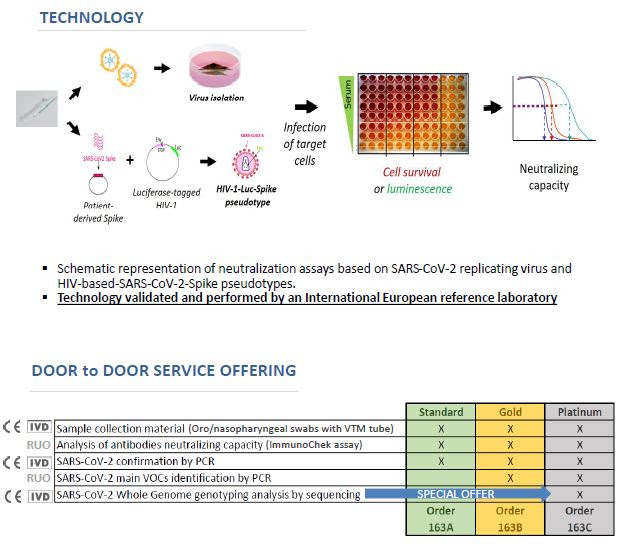
For more information please contact us at the following email address:
contact@ablsa.com
Turnkey Solutions for SARS-CoV-2 Variants Screening (Delta, Lambda, Epsilon…)
UltraGene® & DeepChek® assays for SARS-CoV-2 Variants Screening
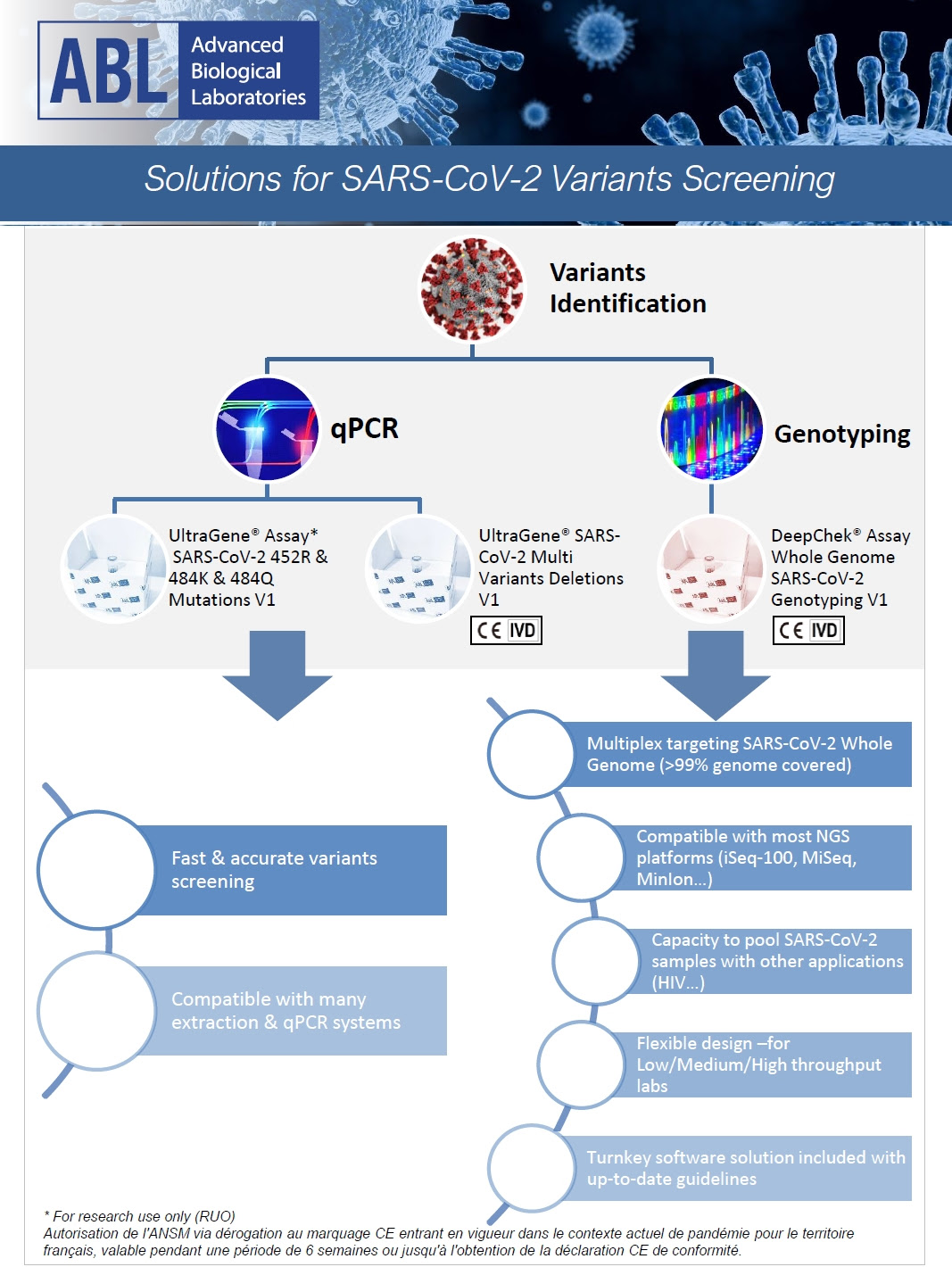
For more information please contact us at the following email address:
contact@ablsa.com
LNS ET ABL DÉVELOPPENT UNE SOLUTION DE DIAGNOSTIC COMMUNE DANS LA LUTTE CONTRE LA COVID-19
Le Laboratoire national de santé (LNS) et Advanced Biological Laboratories (ABL) s’associent dans la lutte contre la COVID-19. A cette fin, les deux laboratoires ont signé un accord qui prévoit le développement d’une solution de diagnostic intégrée. Le projet, appelé INNOV-COV-ID, est cofinancé par le ministère de l’Economie.
Cette collaboration en matière de R&D sera initialement limitée à une période de 30 mois. Le LNS apportera son expertise en tant que laboratoire national de référence pour les infections respiratoires aiguës, un statut qui le place à la pointe de la lutte contre la COVID-19. ABL, basée à Luxembourg-Ville, apportera son expertise dans le domaine des technologies de santé basées sur l’informatique. L’accent sera mis sur le développement et la validation d’une solution intégrée pour un diagnostic rapide, précis et sûr de la COVID-19 à l’aide d’une application mobile marquée CE-IVD.
À la recherche d’une solution intégrée de test rapide
« La pandémie de SRAS-CoV-2 a mis en évidence l’importance de développer de telles solutions », déclare le Dr Tamir Abdelrahman, chef du département de microbiologie du LNS. « Notre objectif est donc de concevoir des applications holistiques qui combinent la commodité de l’autodiagnostic à domicile via des échantillons de salive avec la fiabilité du test PCR, jusqu’à présent réalisé en laboratoire, et l’interprétation ou la communication rapide des résultats. »
Pour atteindre cet objectif, les microbiologistes du LNS s’engagent pour la toute première fois dans un partenariat public-privé, comme le souligne le directeur du LNS, le Pr Dr Friedrich Mühlschlegel : « Le LNS a été, dès le début, à la pointe du combat contre la COVID-19. Nous avons évalué plus de 30 kits pour le diagnostic de la COVID-19. Nous sommes heureux de pouvoir maintenant partager cette expérience, dans l’intérêt de tout le pays, avec un partenaire industriel qui est reconnu, comme le LNS, au-delà des frontières du Luxembourg en tant que leader en matière d’innovation. »
Une innovation pertinente pour le secteur de la santé
Chalom Sayada, PDG et fondateur d’ABL, ajoute : « Cela fait maintenant plus de 20 ans que nous développons des solutions de diagnostic basées sur le web et les logiciels pour le secteur de la santé. Nous nous sommes concentrés sur les maladies infectieuses telles que le VIH, l’hépatite et le HPV, pour lesquelles nous avons établi une clientèle mondiale. Avec la COVID-19, nous avons étendu nos activités pratiquement du jour au lendemain et nous nous réjouissons maintenant de développer l’innovation au service des personnes avec l’un des principaux acteurs de la santé au Luxembourg. »
En plus d’apporter son expertise technologique, ABL se chargera de la coordination du projet. Chalom Sayada : « Après que le LNS nous a approchés avec l’idée d’une collaboration, nous avons immédiatement défini les processus possibles et déposé une demande de financement auprès du ministère de l’Économie dans le cadre du projet INNOV-COV-ID. Celui-ci nous a été accordé très rapidement, si bien que nous sommes aujourd’hui en mesure de lancer conjointement un projet durable et d’une pertinence incontestable. »
Aider à déployer rapidement des mesures efficaces
La question de la pertinence est également centrale pour Françoise Liners, responsable des technologies de la santé au ministère de l’Économie : « Des solutions faciles à utiliser pour détecter une infection aident les acteurs de la santé publique à prendre rapidement et efficacement des mesures appropriées. Nous sommes donc heureux de voir deux leaders de l’écosystème Health Tech luxembourgeois mettre en commun leurs expertises complémentaires pour créer ensemble une technologie qui ne manquera pas d’impressionner au niveau international. Ce projet démontre la maturité de cet écosystème qui contribue à la lutte contre la pandémie de la COVID-19. »
ABL Announces Distribution Agreement with AN BINH MEDICAL in Vietnam
Advanced Biological Laboratories (ABL) S.A. and An Binh Medical are pleased to announce that they entered into an exclusive distribution agreement of ABL’s Diagnostics line of products, including the DeepChek® Assays and software, in Vietnam.
See current channel partners.
COMMUNIQUÉ DE PRESSE – COVID-19 : effervescence d’innovations dans le Grand Est
Première région touchée par la pandémie, le Grand Est est également la première région qui a mobilisé avec succès, depuis le début de la crise sanitaire, l’intégralité de son écosystème santé avec le soutien du pôle de compétitivité BioValley France. La COVID-19 nous prouve aujourd’hui que le
développement et l’implication d’écosystèmes régionaux sont des atouts indispensables dans la reconquête de notre pleine souveraineté sanitaire ; ils doivent être encouragés et soutenus dans le but de renforcer les politiques nationales et de répondre favorablement aux besoins des professionnels de santé, des patients et plus largement des citoyens.

Téléchargez le communiqué de presse ICI
Spring SPECIAL OFFER
New Customer: GET A FREE SAMPLE among one of our genotyping assays
You are a new Client ?
This offer is made for you !!
Get your free sample kit among all our microbiology genotyping assays (SANGER or Next Generation Sequencing)
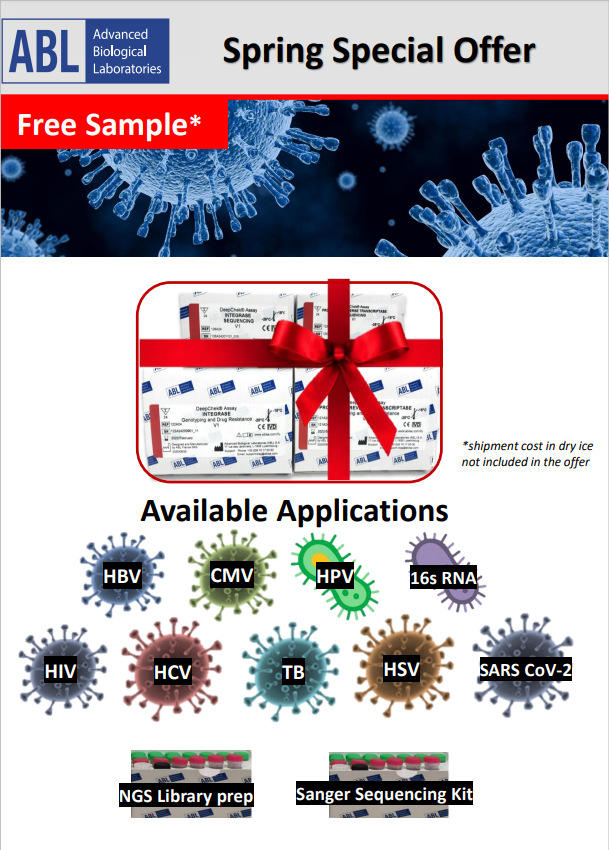
Offer Available Until 30th May, 2021
ABL Announces Distribution Agreement with ROCHE SISTEMAS DE DIAGNÓSTICOS, LDA in Portugal
Advanced Biological Laboratories (ABL) S.A. and Roche Diagnostics are pleased to announce that they entered into an exclusive distribution agreement of ABL’s Diagnostics line of products, including the DeepChek® Assays and software, in Portugal.
See current channel partners.
Innovative And Reliable Turnkey Solution For SARS-CoV-2 Detection And Variants Monitoring


For more information please contact us at the following email address:
contact@ablsa.com
New Kit & Software for SARS-CoV-2 Whole Genome Sequencing
We are pleased to announce the availability of the DeepChek® Assay Whole Genome SARS-CoV-2 Genotyping and the DeepChek® CoV2 Software. Both combined, it offers an innovative end-to-end solution (for Research Use Only (RUO)) for SARS-CoV-2 whole genome sequencing and analysis: from SARS-CoV-2 whole-genome amplification, library preparation, high-throughput sequencing (Next Generation Sequencing, (NGS)) to genotyping and downstream software analysis with publicly available knowledge reference databases. The solution lists mutations, insertions and deletions to then identify circulating SARS-CoV-2 variants (VOCs) using RNA extracted from patients already diagnosed positive to SARS-CoV-2.
In continuation of the NGS sequencing, the software suite allows an advanced bioinformatic analysis of the raw sequencing data before the analysis and the reporting of latest epidemiological and clinical knowledge linked to the identified mutations, insertions and deletions (i.e., determination of the risk of infectivity or vaccine escape).
The DeepChek® Assay Whole Genome SARS-CoV-2 Genotyping combined with the DeepChek® CoV2 Software allow laboratories performing PCR and next generation sequencing to sequence the entire genome of the SARS-CoV-2. It is an additional technique to qPCR which generates information necessary to better control and understand the emergence of variants in general and more specifically on the main circulating variants of concern with their mutations of interest (i.e. the Spike E484K, N501Y…).
The whole-genome sequencing is to be used only in second intention on SARS-CoV-2 samples already diagnosed positive by an approved RT-PCR or RT-LAMP device.


For more information please contact us at the following email address:
contact@ablsa.com
From Sample to Result in HIV Genotyping with the DeepChek®-HIV Solution
We are pleased to announce the immediate availability of the DeepChek®-HIV Solution combining CE-IVD target-specific amplification assays with data interpretation software both compatible with SANGER and Next Generation Sequencing (NGS) platforms.


For more informations or to build a project for your laboratory, please contact us at the following email address:
contact@ablsa.com
Availability of a full range of molecular biology instruments for SARS-CoV-2 detection and more
We are pleased to announce the immediate availability of robust, cost-effective & open instruments for SARS-CoV-2 detection and other applications.

CleanPrepX : a magnetic bead-based automated extractor for all your samples
- Manual or automated workflow
- Template compatibility : Serum, plasma, whole blood, Oro/naso swabs, respiratory specimens…
- 96 preps within 24 minutes
- Excellent purity (A260/280 < 1.7 ; A260/230 > 1.5)
- Dowstream application : qPCR (detection), NGS (genotyping) …
UltraGene qPCR / PCR : high performance instruments
- qPCR instrument at 48 or 96 format
- PCR instrument at 96 or 384 format
- 4 fluorescent channels with thermal gradient
- Reaction volume : 5 – 100µL
- Temperature accuracy & Temperature uniformity : 0.1°C
- Heating rate : 8.0°C/s
- Cooling rate : 5.5°C/s
DeepChek Titanium : an accurate, compact and affordable pipetting platform
- Application covered : qPCR, PCR cleanup, NGS, normalization …
- Active magnetic module
- Handle 96 and 384-well plates
- User-friendly software
- Pipetting quality better than recommendation of ISO 8655 : CV < 5% for 1µL and CV < 0.25% for 200µL
Tips / Plates / Tubes : consumables at your fingertips
- Plastic for running your PurePrep 96 instrument – bulk of 5000 preps
- Microplates/strips of tubes fully validated on our qPCR instrument
- Tips with/without filter, fully compatible with our pipetting platform and designed for advoid cross-contamination
For more informations or to reserve your instrument, please contact us at the following email address:
contact@ablsa.com
New Publication on DeepChek®-HCV Drug Resistance Assays and Software (Virology Journal)
Detection of low-level HCV variants in DAA treated patients: comparison amongst three different NGS data analysis protocols
Caputo et al. Virology Journal (2020) 17:103
https://doi.org/10.1186/s12985-020-01381-3
Abstract
Background: Notwithstanding the efforts of direct-acting antivirals (DAAs) for the treatment of chronically infected
hepatitis C virus (HCV) patients, concerns exist regarding the emergence of resistance-associated substitutions (RAS)
related to therapy failure. Sanger sequencing is still the reference technique used for the detection of RAS and it
detects viral variants present up to 15%, meaning that minority variants are undetectable, using this technique. To
date, many studies are focused on the analysis of the impact of HCV low variants using next-generation sequencing
(NGS) techniques, but the importance of these minority variants is still debated, and importantly, a common data
analysis method is still not defined.
Methods: Serum samples from four patients failing DAAs therapy were collected at baseline and failure, and
amplification of NS3, NS5A and NS5B genes was performed on each sample. The genes amplified were sequenced
using Sanger and NGS Illumina sequencing and the data generated were analyzed with different approaches. Three
different NGS data analysis methods, two homemade in silico pipeline and one commercially available certified
user-friendly software, were used to detect low-level variants.
Results: The NGS approach allowed to infer also very-low level virus variants. Moreover, data processing allowed to
generate high accuracy data which results in reduction in the error rates for each single sequence polymorphism.
The results improved the detection of low-level viral variants in the HCV quasispecies of the analyzed patients, and
in one patient a low-level RAS related to treatment failure was identified. Importantly, the results obtained from
only two out of the three data analysis strategies were in complete agreement in terms of both detection and
frequency of RAS.
Conclusions: These results highlight the need to find a robust NGS data analysis method to standardize NGS results
for a better comprehension of the clinical role of low-level HCV variants. Based on the extreme importance of data
analysis approaches for wet-data interpretation, a detailed description of the used pipelines and further standardization
of the in silico analysis could allow increasing diagnostic laboratory networking to unleash true potentials of NGS.
ABL Appointed as the Exclusive Distributor of all NimaGen sequencing products for France, Belgium and Luxembourg
We are pleased to announce that Nimagen appointed ABL as its exclusive distribution partner for Sequencing Products in France, Belgium and Luxembourg.
The partnership was formalized on March 17th 2020 and will help all labs involved in sequencing to get access to robust and cost-effective solutions including the BrilliantDye™ Terminator Sequencing Kits, the iX-Pure™ DyeTerminator Cleanup kits… as well as solutions for Next-Generation Sequencing including AmpliClean™ Cleanup kits.
Please contact the ABL Team for any inquiries or questions:
E-mail: contact@ablsa.com
Phone: +352 263 896 761
Advanced Biological Laboratories Receives CE-IVD Registration for its SANGER and for its NGS DeepChek®-HIV Genotyping Drug Resistance Assays
A unique and flexible solution adapted to all virology and microbiology laboratories
LUXEMBOURG CITY (Luxembourg), METZ (France) — May 18th, 2020 — Advanced Biological Laboratories (ABL) announced today the CE-IVD marking of its DeepChek®-HIV assays, now available for in-vitro diagnostics. Intended to be used on HIV-1 Group M viruses from patients diagnosed with HIV infection, the assays deliver standardized, open and flexible solution suited to clinical settings performing sequencing through Capillary Electrophoresis and Next Generation Sequencing (NGS) systems.
The DeepChek®-HIV CE IVD Assays are covering respectively the Protease / Reverse Transcriptase and the Integrase regions of the virus and are intended to be used from input RNA extracted from plasma, serum or whole blood samples. Both assays are highly sensitive and have been validated to process clinical samples as low as 1,000 copies/mL with outstanding performances (100% agreement of analytical reproducibility and repeatability, 100% clinical reproducibility, 99% clinical sensitivity) in all three regions.
Open and flexible, the DeepChek®-HIV CE IVD Assays is a unique and versatile system that can be used under a large variety of laboratory throughput configurations.
“Obtaining CE IVD marking for our DeepChek®-HIV assays will allow virology labs to access a unique and innovative technology, for HIV genotyping diagnostics. ABL will keep standardizing its entire portfolio of applications in virology and microbiology following European and International guidelines to improve the management of patients suffering from chronic diseases on a worldwide basis.” said Dr. Chalom Sayada, CEO of ABL.
HIV remains a major public health threat throughout in developed and developing countries. “We are extremely pleased to offer a growing portfolio of standardized diagnostics applications for infectious diseases to our clients and partners, around RNA or DNA detection (UltraGene®) and for sequencing-based genotyping (DeepChek®)”, explained Dimitri Gonzalez, Head of the Diagnostics division of ABL.
“The DeepChek®-HIV CE IVD Assays are very flexible and perfectly suited to a broad range of settings running either Sanger or NGS workflows. They have for instance been validated together with the DeepChek® Library Preparation assays on several NGS platforms from Illumina including the iSeq100 instrument.”, added Dr. Sofiane Mohamed, Head of Laboratory Research and Development.
“The regulatory efforts ABL has engaged, for certifying its molecular diagnostics applications in continuation of the work around SARS-CoV-2, needed to be taken for the potential benefits of safety and efficacy to the HIV patients.”, added Mr. Ronan Boulmé, Governance, Risk and Compliance Manager of the ABL Group.
To learn more about DeepChek® solutions and test menu, please visit https://www.ablsa.com/laboratory-solutions/deepchek-assays/
# # #
About Advanced Biological Laboratories (ABL SA)
Improving Disease Management
Advanced Biological Laboratories (ABL), S.A., is a diagnostic and medical software company founded in 2000 as a spin-off from CRP-Santé (https://www.lih.lu/) Luxembourg.
ABL’s products offer to infectious disease clinicians, virology and microbiology laboratories
- Assays and standalone software systems for accredited laboratories (i.e. ISO 15189), mainly for microbiology applications (related to HIV, Coronavirus, Tuberculosis, HCV, HBV, HPV, CMV, HPV, Flu, 16s RNA…) for clinical genotyping through sequencing (DeepChek®), DNA and RNA detection and quantification (UltraGene®), including powerful downstream analysis software applications fully integrated with knowledge databases and analysis systems for capillary and high-throughput Next Generation Sequencing data.
- Clinical software applications for infectious diseases units
- IT dashboards and clinical database aggregation applications for research and clinical management
ABL took in 2013 the rights to all viral hepatitis B & C related assets from EVIVAR MEDICAL as well as a personalized medicine electronic medical record system (EMR) in infectious disease from GlaxoSmithKline in 2016. In July 2018, acquired CDL Pharma to develop CRO related services and assays manufacturing capacity. In June 2019, ABL created its affiliate in the USA (AdvancedDx Biological Laboratories) covering the entire North American territory.
ABL has a comprehensive suite of healthcare management products, including Nadis®, TherapyEdge®, ViroScore®, SeqHepB, DeepChek®, UltraGene®, VisibleChek®, HepatiC®, BacterioChek, MicrobioChek and the DPM used for data and patient management, monitoring and personalized reporting applications. Since 2012, some of ABL’s products are CE-IVD marked. In 2020, ABL got CE-IVD marking for its DeepChek®-HIV assays as well as for its UltraGene Combo2ScreenSARS-CoV-2 assay. The other products are currently available for Research Use Only.
For more information, visit www.ablsa.com.
Media Contact:
Advanced Biological Laboratories
contact@ablsa.com
+352 263896761















