Description
The DeepChek® Assay product line combines target-specific PCR reagents with in vitro diagnostic software both compatible with either Sanger or Next Generation Sequencing platforms for Microbiology or Virology applications (like HIV, Hepatitis C, Hepatitis).
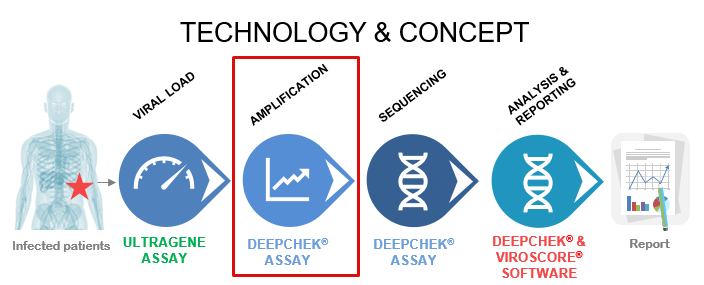
Each assay is suited to 24 sample analyses and contains all the RT-PCR or PCR reagents to perform a targeted amplification of the key actionable genes (Ex.: RT/PROT for HIV, NS5B/5’UTR for HCV genotyping, NS5A/NS3/NS5B for HCV Drug Resistance…). The assay also includes Sanger sequencing primers, detailed protocols for Sanger or the main Next Generation Sequencing platforms and the software for Sanger or NGS sequencing data analysis and interpretation.
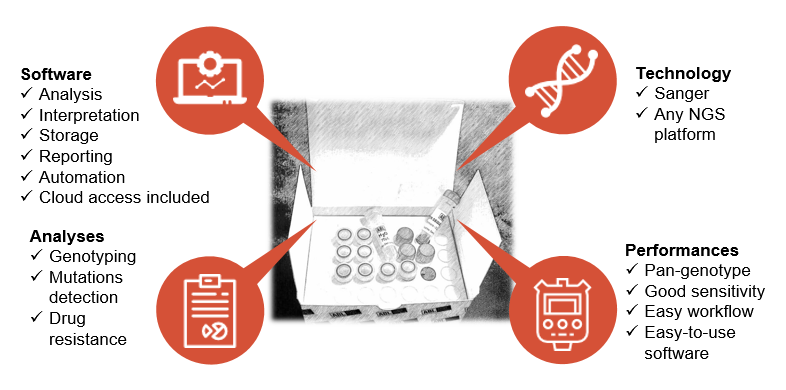
Characteristics and performances
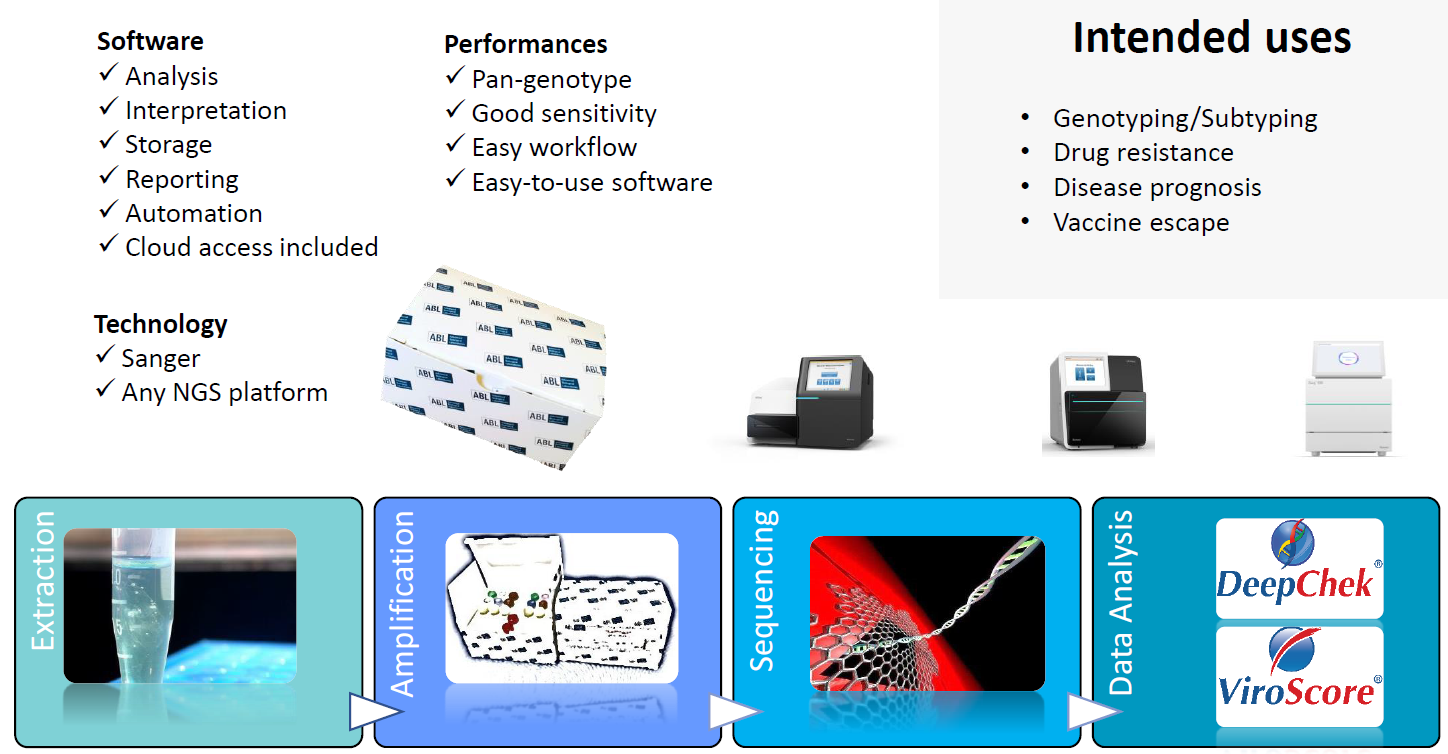
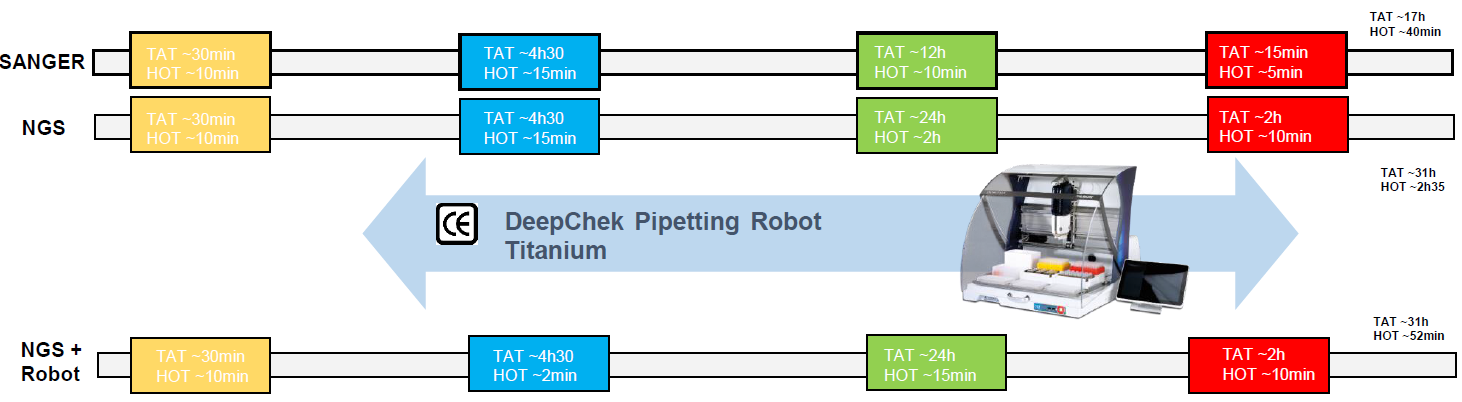
Overview of the workflow
Examples of report

Downloads
General documentation
Posters
-
Evaluation of a sequence-based method for hepatitis C genotyping (DeepChek® SingleRound PCR and NS5B/5'UTR sequencing) ...
-
Retreatment with Direct Active Antivirals of Genotype 1, 3 and 4 Chronic Hepatitis C Patients who Previously Failed an Anti-NS5A-Containing Regimen in Real World
-
Prevalence and Characterization of NS5A Resistance Associated Variants (RAVs) in Patients Who Relapsed [...]
-
Novel End-to-end Sequencing Solutions for Sanger and Next Generation Sequencing (NGS) of HIV and Viral Hepatitis C (HCV)
-
Robust Viral Hepatitis C Subtyping through DeepChek HCV NS5B Assay
-
Validation of “DeepChek SingleRound® RT-PCR and Sequencing PR/RT Assay v.2” kit as HIV-1 Drug Resistance Test for routine laboratory
Papers
-
Hepatitis C Genotype 4R Resistance-Associated Polymorphisms: The Achilles Heel of the Nonstructural 5A Inhibitors?
-
Clinical relevance of the HCV protease inhibitor-resistant mutant viralload assessed by ultra-deep pyrosequencing in treatment failure
