
Description
DeepChek® is a downstream analysis software developed by ABL, able to handle sequencing data and intended to be used for performing genomic data analyses (genotyping, subtyping, amino-acid mutations detection, nucleotide changes characterization…) and clinical interpretations (drug resistance, vaccine escape, disease prognosis…).
Compatible with Electrophoresis Capillary and Next Generation Sequencing (NGS) platforms, DeepChek® is available for different applications (HIV, HBV, HCV, CMV, HPV…), stores, organizes sequencing data in a dedicated database format. Depending on the application, on the type of sequencing platform and based on the requirements from end users, it performs several types of analyses, interpretations and is able to generate reports to be used either for research or for routine use.
- Supported formats: AB1, FASTA and PLAIN-TEXT for Sanger, FASTA/FASTQ (including paired sequencing), BAM/SAM for NGS covering Targeted Genes or also compatible with Whole Genome data.
- Examples of analyses: genotyping, high-resolution subtyping, variants calling…
- Examples of interpretations: drug resistance, disease prognosis, …
- Included services: updates/upgrades (quarterly releases), support, web-training, historical sequencing data import
- On-demand services: local training, integration with third-party instruments or systems, customization…
The DeepChek® software system is a secured web application which can be used through a Cloud access or locally, through pre-configured servers. It is made available with regular updates (new clinical databases, guidelines…) and quarterly upgrades (new features, modules, applications…) and can be fully integrated within the IT network of each laboratory (integration with the sequencing platform, with the Laboratory Information System – LIS, with the Hospital Information System – HIS…).
For “In-Vitro Diagnostic use (IVD)” where indicated : CE marking only valid for EEA and territories recognizing it. Otherwise “For Research Use Only” (RUO): not for use in diagnostic procedures, no claim or representation is intended to provide information for the diagnosis, prevention, or treatment of disease.
Limitations
1. DeepChek® Software is a downstream analysis software program (“Program“) which enables virologists to input pre-formatted sequences from genetic analyzers (Sanger or Next Generation Sequencing) using either CE-IVD genotyping assays (“IVD information”) or for Research Use Only (“RUO information”) genotyping assays (“PCR amplification”) in order to obtain viral or bacterial sequence analysis and viral or bacterial drug resistance interpretations or other interpretations to adapt accordingly patient’s anti-viral or anti-bacterial drugs (“Analyses“).
2. For In Vitro Diagnostic Use only with IVD information or with combination of IVD information and RUO information. For research use only with RUO information alone.
3. Responses to anti-viral and anti-bacterial treatment are complex and affected by a number of factors not taken into account by the Program.
4. The selection of drugs for the treatment of viral or bacterial infection is the responsibility of the physician in consultation with the patient and reliance should not be placed on the Analyses only for such purposes.
5. The Analyses are not intended to replace professional medical care and attention by a qualified medical practitioner and consequently ABL does not accept any responsibility for the selection of drugs and the patient’s response to treatment.
6. As the accuracy of the results highly depends on the sequencing technology and on its related technical recommendations (Ex.: In case of ‘low’ viral load input, the users should be aware of risks of resampling errors), ABL cannot take any responsibility on the reliability of the results if the recommendations of the suppliers are not strictly followed.
7. ABL does not accept any responsibility for the accuracy of the data entered by the user or the consequences of any inaccuracies in those data.
Overview of the pipeline
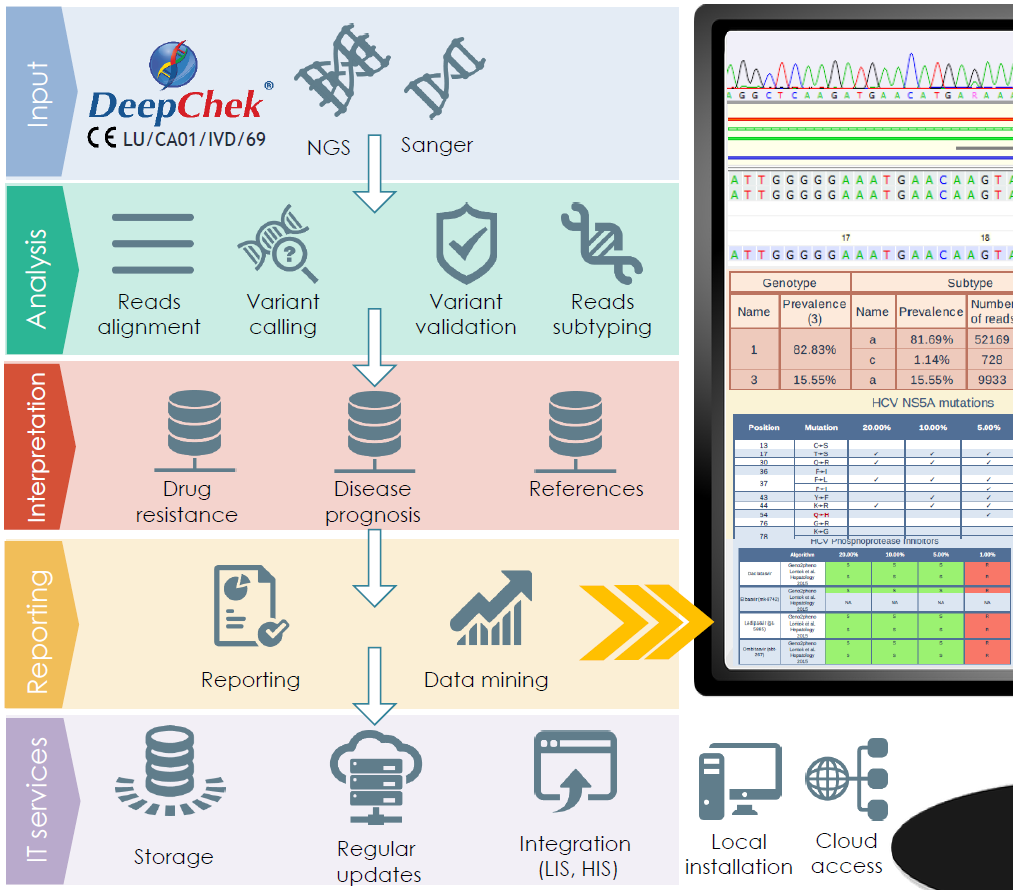
Examples of reports

Downloads
General documentation
Posters
-
Implementation of Next-Generation Sequencing for Hepatitis B Resistance and Genotyping in a Clinical Laboratory
-
Use of Next-Generation Sequencing Technologies in the Clinical HIV Laboratory
-
Next Generation Sequencing for HIV virus genotyping and detection of antiviral resistance
-
Clinical impact of Ultra-Deep Versus Sanger Sequencing detection of minority mutations on the HIV-1 [...]
-
An End-To-End Solution in Deep Sequencing of HIV: Sensitive Detection of HIV-1 Genomic Variations Combined with [...]
-
Hepatitis C Virus High Resolution Subtyping Using Next Generation Sequencing (NGS) Data
-
A Novel Software and Database Solution Tool for Analysis of Sanger and Next Generation Sequencing (NGS)
-
Advanced Medical Devices for HCV High Resolution Subtyping, Genotyping and Drug Resistance Testing [...]
-
Use of Illumina MiSeq Technology to Detect Drug Resistance Mutations in Human Cytomegalovirus
-
Use of DeepChek® v1.1 and VisibleChek® for the analysis and integration of 454 GS Junior data from the RT and Protease of HIV-1
-
Advanced Medical Devices for HBV Genotyping, Drug Resistance Testing and Detection of Surface Antigen Mutants Using [...]
-
Next-Generation Sequencing Technology in the Clinical HIV Laboratory: A More Sensitive Alternative to Sanger Sequencing
-
DeepChek® HIV v1.0., a reliable tool for the bioinformatics analysis and resistance interpretation of Massive Ultra Deep [...]
-
Detection of Minority HIV-1 Drug-Resistant Variants Moderately Improves the Prediction of Salvage Antiretroviral [...]
-
Added value of Ultra Deep Sequencing in patients with HIV-1 Transmitted Drug Resistance mutations [...]
-
A Fully Integrated and Simplified HIV Clinical Genotyping Solution Using 454 Ultra-Deep-Sequencing and [...]
Papers
-
Minority resistant HIV-1 variants and the response to first-line NNRTI therapy
-
High-Resolution Hepatitis C Virus Subtyping Using NS5B Deep Sequencing and Phylogeny, an Alternative to Current Methods
-
Comparison of ultra-deep versus Sanger sequencing detection of minority mutations on the HIV-1 drug resistance [...]
-
COMET: adaptive context-based modeling for ultrafast HIV-1 subtype identification
-
Evaluation of GS Junior and MiSeq next-generation sequencing technologies as an alternative to Trugene [...]
