ABL Appointed as the Exclusive Distributor of all NimaGen sequencing products for France, Belgium and Luxembourg
We are pleased to announce that Nimagen appointed ABL as its exclusive distribution partner for Sequencing Products in France, Belgium and Luxembourg.
The partnership was formalized on March 17th 2020 and will help all labs involved in sequencing to get access to robust and cost-effective solutions including the BrilliantDye™ Terminator Sequencing Kits, the iX-Pure™ DyeTerminator Cleanup kits… as well as solutions for Next-Generation Sequencing including AmpliClean™ Cleanup kits.
Please contact the ABL Team for any inquiries or questions:
E-mail: contact@ablsa.com
Phone: +352 263 896 761
Advanced Biological Laboratories Receives CE-IVD Registration for its SANGER and for its NGS DeepChek®-HIV Genotyping Drug Resistance Assays
A unique and flexible solution adapted to all virology and microbiology laboratories
LUXEMBOURG CITY (Luxembourg), METZ (France) — May 18th, 2020 — Advanced Biological Laboratories (ABL) announced today the CE-IVD marking of its DeepChek®-HIV assays, now available for in-vitro diagnostics. Intended to be used on HIV-1 Group M viruses from patients diagnosed with HIV infection, the assays deliver standardized, open and flexible solution suited to clinical settings performing sequencing through Capillary Electrophoresis and Next Generation Sequencing (NGS) systems.
The DeepChek®-HIV CE IVD Assays are covering respectively the Protease / Reverse Transcriptase and the Integrase regions of the virus and are intended to be used from input RNA extracted from plasma, serum or whole blood samples. Both assays are highly sensitive and have been validated to process clinical samples as low as 1,000 copies/mL with outstanding performances (100% agreement of analytical reproducibility and repeatability, 100% clinical reproducibility, 99% clinical sensitivity) in all three regions.
Open and flexible, the DeepChek®-HIV CE IVD Assays is a unique and versatile system that can be used under a large variety of laboratory throughput configurations.
“Obtaining CE IVD marking for our DeepChek®-HIV assays will allow virology labs to access a unique and innovative technology, for HIV genotyping diagnostics. ABL will keep standardizing its entire portfolio of applications in virology and microbiology following European and International guidelines to improve the management of patients suffering from chronic diseases on a worldwide basis.” said Dr. Chalom Sayada, CEO of ABL.
HIV remains a major public health threat throughout in developed and developing countries. “We are extremely pleased to offer a growing portfolio of standardized diagnostics applications for infectious diseases to our clients and partners, around RNA or DNA detection (UltraGene®) and for sequencing-based genotyping (DeepChek®)”, explained Dimitri Gonzalez, Head of the Diagnostics division of ABL.
“The DeepChek®-HIV CE IVD Assays are very flexible and perfectly suited to a broad range of settings running either Sanger or NGS workflows. They have for instance been validated together with the DeepChek® Library Preparation assays on several NGS platforms from Illumina including the iSeq100 instrument.”, added Dr. Sofiane Mohamed, Head of Laboratory Research and Development.
“The regulatory efforts ABL has engaged, for certifying its molecular diagnostics applications in continuation of the work around SARS-CoV-2, needed to be taken for the potential benefits of safety and efficacy to the HIV patients.”, added Mr. Ronan Boulmé, Governance, Risk and Compliance Manager of the ABL Group.
To learn more about DeepChek® solutions and test menu, please visit https://www.ablsa.com/laboratory-solutions/deepchek-assays/
# # #
About Advanced Biological Laboratories (ABL SA)
Improving Disease Management
Advanced Biological Laboratories (ABL), S.A., is a diagnostic and medical software company founded in 2000 as a spin-off from CRP-Santé (https://www.lih.lu/) Luxembourg.
ABL’s products offer to infectious disease clinicians, virology and microbiology laboratories
- Assays and standalone software systems for accredited laboratories (i.e. ISO 15189), mainly for microbiology applications (related to HIV, Coronavirus, Tuberculosis, HCV, HBV, HPV, CMV, HPV, Flu, 16s RNA…) for clinical genotyping through sequencing (DeepChek®), DNA and RNA detection and quantification (UltraGene®), including powerful downstream analysis software applications fully integrated with knowledge databases and analysis systems for capillary and high-throughput Next Generation Sequencing data.
- Clinical software applications for infectious diseases units
- IT dashboards and clinical database aggregation applications for research and clinical management
ABL took in 2013 the rights to all viral hepatitis B & C related assets from EVIVAR MEDICAL as well as a personalized medicine electronic medical record system (EMR) in infectious disease from GlaxoSmithKline in 2016. In July 2018, acquired CDL Pharma to develop CRO related services and assays manufacturing capacity. In June 2019, ABL created its affiliate in the USA (AdvancedDx Biological Laboratories) covering the entire North American territory.
ABL has a comprehensive suite of healthcare management products, including Nadis®, TherapyEdge®, ViroScore®, SeqHepB, DeepChek®, UltraGene®, VisibleChek®, HepatiC®, BacterioChek, MicrobioChek and the DPM used for data and patient management, monitoring and personalized reporting applications. Since 2012, some of ABL’s products are CE-IVD marked. In 2020, ABL got CE-IVD marking for its DeepChek®-HIV assays as well as for its UltraGene Combo2ScreenSARS-CoV-2 assay. The other products are currently available for Research Use Only.
For more information, visit www.ablsa.com.
Media Contact:
Advanced Biological Laboratories
contact@ablsa.com
+352 263896761
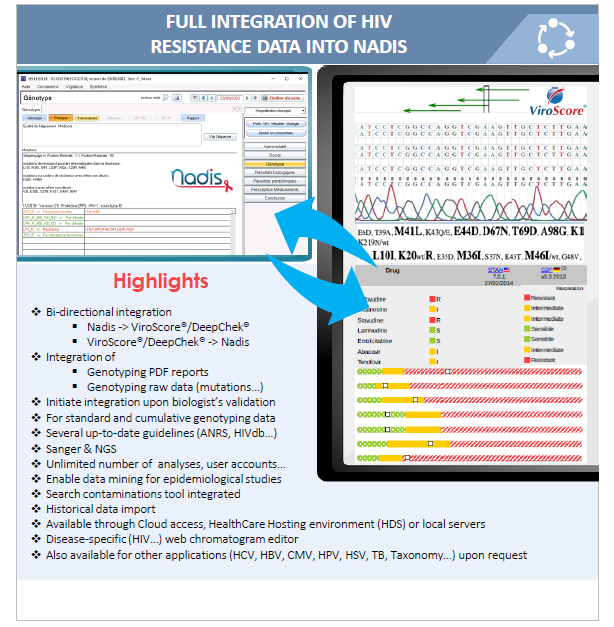
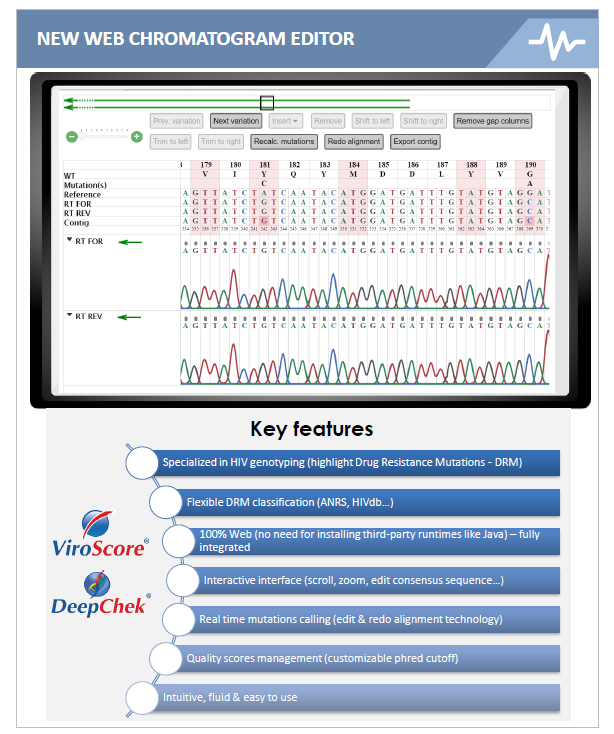
Major Updates on HIV Genotyping
ABL is pleased to announce two major updates on its HIV Genotyping Software applications :
- the full integration of the ViroScore® and DeepChek® software applications with Nadis
- the availability of a new web-based and disease-specific chromatogram editor

Meet ABL at MEDICA for Leveraging your Infectious Diseases Genotyping Capacities?
Meet us at MEDICA 2019 for Leveraging your Infectious Diseases Genotyping Capacities
Good morning,
ABL will be attending the next MEDICA forum in Düsseldorf from 18 – 21 November 2019 (Hall 3 / A74).
We will be pleased to have a chance to meet you there and explain you in detail the SAMPLE-TO-RESULT solutions ABL can offer in INFECTIOUS DISEASES GENOTYPING.
Thank you.
With best regards,
The ABL Diagnostics Team
TGEN AND ABL PLAN GLOBAL ROLLOUT OF ADVANCED TEST FOR TB, ONE OF THE WORLD’S MOST DEADLY PATHOGENS
DeepChek®-TB will help enable modern medicine to thwart TB in every corner of the globe
FLAGSTAFF, Ariz., and LUXEMBOURG CITY, Luxembourg — Aug. 31 — Advanced Biological Laboratories (ABL) is expected to soon begin manufacturing of a compact, portable and affordable test for tuberculosis (TB), designed by the Translational Genomics Research Institute (TGen), an affiliate of City of Hope.
Under an expanded licensing agreement with TGen announced today, ABL will make, market and distribute this state-of-the-art TB test, which now covers the known important genes and mutations that cause resistance to current anti-TB drugs. This will help physicians determine the most appropriate treatment for each patient of TB, the world’s leading cause of death from a single infectious agent.
For now, this patented Next Generation Sequencing based TB test — called DeepChek®-TB — is available for research use only. The test is based on years of development at TGen, involving numerous research partners from across the globe.
Thanks to modern medicine, TB in the U.S. continues to be a relatively minor threat. Globally, however, nearly one-fourth of the world’s population is infected with this lung-damaging communicable disease, which is estimated to kill nearly 1.3 million people annually, according to the U.S. Center for Disease Control and Prevention (CDC).
“The continued partnership between TGen and ABL is allowing for next generation science to be applied to patients suffering from the most important infectious disease of our time,” said Dr. David Engelthaler, Co-Director of TGen’s Pathogen and Microbiome Division. “The combination of TGen’s scientific advancements and ABL’s manufacturing and distribution channels means that someday soon people most at risk, in every corner of the globe, will be able to access the most advanced TB medical information.”
TB remains a major public health threat throughout developing nations and is increasing in some places as mutant versions of this disease become resistant to current drug treatments. Identifying rapidly mutating, drug-resistant strains of TB is one of the greatest challenges to eradicating this disease.
ABL, based in Luxembourg, is a leading integrated diagnostics and medical technology company. Its licensing agreement with TGen will enable ABL to distribute DeepChek®-TB through its worldwide network of clinicians and distributors in more than 80 countries.
“We are extremely pleased to upgrade our DeepChek®-TB test and cover additional resistance-associated mutations, and offer to microbiologists the capacity to perform comprehensive drug susceptibility testing on an exhaustive list of drugs, such as Capreomycin, Streptomycin, Quinolones, Pyrazinamide, Ethambutol, Kanamycin and Linezolid. Considering the extremely simple workflow of the solution, which does not require any library preparation, we expect a strong demand for this test from leading research facilities worldwide,” said Dimitri Gonzalez, Head of Diagnostics at ABL.
# # #
About TGen, an affiliate of City of Hope
Translational Genomics Research Institute (TGen) is a Phoenix, Arizona-based non-profit organization dedicated to conducting groundbreaking research with life-changing results. TGen is affiliated with City of Hope, a world-renowned independent research and treatment center for cancer, diabetes and other life-threatening diseases: www.cityofhope.org. This precision medicine affiliation enables both institutes to complement each other in research and patient care, with City of Hope providing a significant clinical setting to advance scientific discoveries made by TGen. TGen is focused on helping patients with neurological disorders, cancer, diabetes and infectious diseases through cutting-edge translational research (the process of rapidly moving research toward patient benefit). TGen physicians and scientists work to unravel the genetic components of both common and complex rare diseases in adults and children. Working with collaborators in the scientific and medical communities worldwide, TGen makes a substantial contribution to help our patients through efficiency and effectiveness of the translational process. For more information, visit: www.tgen.org. Follow TGen on Facebook, LinkedIn and Twitter @TGen.
Media Contact:
Steve Yozwiak
TGen Senior Science Writer
602-343-8704
syozwiak@tgen.org
About Advanced Biological Laboratories (ABL SA)
Advanced Biological Laboratories (ABL), S.A., is a Diagnostic and Medical Software company founded in 2000 as a spin-off from CRP-Santé Luxembourg. ABL’s products offer to infectious disease clinicians, virology and microbiology laboratories optimal and efficient solutions (assays and software systems related to HIV, Tuberculosis, HCV, HBV, HPV, CMV, HPV, Flu, 16s RNA…) for sequencing (DeepChek®) clinical genotyping, viral load (UltraGene™) and drug resistance analysis, including powerful fully integrated databases and analysis systems that combine standard and high-throughput Next Generation Sequencing data. ABL took control of TherapyEdge, Inc. in 2004 and in 2013 the rights to all viral hepatitis B & C related assets from EVIVAR MEDICAL as well as a personalized medicine electronic medical record system (EMR) in infectious disease from GlaxoSmithKline in 2016. In July 2018, acquired CDL Pharma to develop CRO related services and assays manufacturing capacity. In March 2019, ABL signed a non-exclusive global licensing agreement to distribute miRpredX™, IntegraGen’s proprietary IVD CE marked diagnostic test that enables clinicians to identify metastatic colorectal cancer patients who have a higher likelihood of response to anti-EGFR therapy. ABL has a comprehensive suite of healthcare management products, including Nadis®, TherapyEdge®, ViroScore®, SeqHepB, DeepChek®, UltraGene®, VisibleChek®, HepatiC®, BacterioChek, MicrobioChek and the DPM used for data and patient management, monitoring and personalized reporting applications. In 2012, some of ABL’s products also received CE-marking for IVD use. ABL’s products like ViroScore® Suite and DeepChek® are for research use only in the United States, and the data processing module is an FDA registered class I medical device. These are currently available for Research Use Only.
Media Contact:
Dimitri Gonzalez
Advanced Biological Laboratories
contact@ablsa.com
+352 263896761
Connexion du logiciel ViroScore®-HIV (CE IVD) à Nadis disponible
Nous avons le plaisir de vous annoncer que l’intégration du logiciel ViroScore®-HIV (CE IVD) à la plateforme Nadis est disponible. Cette intégration permet à l’ensemble des utilisateurs Nadis de bénéficier de fonctionnalités avancées pour le génotypage du VIH:
- Synchronisation bidirectionnelle des données de résistance (sous-types, mutations, résistance, tropisme…) entre Nadis et ViroScore®-HIV
- Résistance aux antirétroviraux via un ou plusieurs algorithmes (ANRS, HIVdb, Rega, Geno2pheno…) continuellement à jour
- Génotypage classique ou cumulé/longitudinal
- Edition de chromatogrammes intégrée
- Outil de recherche de contaminations
- Outil de data mining pour interroger votre base de données
- Services inclus : import de vos données historiques, personnalisation de vos rapports, formations…
- Différents modèles d’installation pour ViroScore®-HIV: serveur local, hébergement Cloud, hébergement HDS…
- Accès facilité à d’autres paramètres (Sanger/NGS): VHC, VHB, CMV, HPV, HSV, Tuberculose, ARN 16s…
Pour les laboratoires réalisant leurs analyses de génotypage via séquençage NGS, une intégration avec le logiciel DeepChek®-HIV (CE IVD) sera également disponible très prochainement.
Le logiciel ViroScore®-HIV est mis à disposition sous forme de license annuelle illimitée (nombre d’analyses, nombre d’utilisateurs par centre…) et qui peut également être combiné à une offre complète pour le génotypage incluant notamment des kits, des séquenceurs (NGS/Sanger)…
N’hésitez pas à nous contacter pour avoir plus d’informations.
L’équipe ABL et Fedialis
ABL Announces Distribution Agreement with Hydrox SIA in Latvia
Advanced Biological Laboratories (ABL) S.A. and ILC – Hydrox SIA are pleased to announce that they entered into an exclusive distribution agreement of ABL’s Diagnostics line of products, including the DeepChek® Assays and software, in Latvia.
See current channel partners.
Connection du logiciel ViroScore®-HIV (CE IVD) à Nadis bientôt disponible
Nous avons le plaisir de vous annoncer que l’intégration du logiciel ViroScore®-HIV (CE IVD) à la plateforme Nadis est bientôt disponible. Cette intégration va permettre à l’ensemble des utilisateurs Nadis de bénéficier de fonctionnalités avancées pour le génotypage du VIH telles que l’accès à un éditeur de chromatogrammes, la possibilité de générer des rapports de résistance avec différentes bases de connaissances constamment à jour sur l’ensemble des antirétroviraux, l’accès à du génotypage longitudinal, à un outil de recherche de contaminations, au stockage des séquences dans la base Nadis…
Pour les laboratoires réalisant leurs analyses de génotypage via séquençage NGS, une intégration avec le logiciel DeepChek®-HIV (CE IVD) est également envisageable sur demande.
N’hésitez pas à nous contacter pour avoir plus d’informations et demande de souscription. Nous sommes également en mesure de vous offrir des solutions d’intégration pour d’autres applications telles que les hépatites virales, la tuberculose, le cytomégalovirus, la taxonomie bactérienne via ARN 16s…
L’équipe ABL et Fedialis
MicrobioChek – An innovative software system for micro-organisms analysis and clinical interpretation
ABL is pleased to announce the development and future release of the first version of MicrobioChek, a generic downstream analysis software intended to be used by microbiology laboratories for micro-organism related molecular analysis.
Developed with the support of a RDI grant from the Ministry of the Economy in Luxembourg, this innovative tool will leverage ABL efforts toward the development and commercialization of generic end-to-end platforms used for optimizing the management of infectious diseases in the new era of Personalized Disease, Precision Medicine and optimal use of Molecular biomarkers.
TGen and ABL pursue global rollout of advanced TB test
Licensing agreement puts DeepChek®-TB one step closer to clinical application
FLAGSTAFF, Ariz., LUXEMBOURG City, Luxembourg — In an important step toward eradicating tuberculosis (TB), the Translational Genomics Research Institute (TGen), an affiliate of City of Hope, has signed a licensing agreement with an international biomedical firm, Advanced Biological Laboratories (ABL), to market and distribute TGen’s patented and Next Generation Sequencing based TB test technology.
See the related Press Release
Advanced Biological Laboratories, Mayo Clinic Laboratories collaborate on test development to help patients with cytomegalovirus infection
LUXEMBOURG VILLE, Luxembourg, and ROCHESTER, Minn. – Advanced Biological Laboratories (ABL), S.A., a Luxembourg-based diagnostics company and leader in virology genotyping, and Mayo Clinic Laboratories have announced a collaboration. The two organizations are working together to develop a clinical test that will detect mutations associated with antiviral resistance in human cytomegalovirus.
See the related Press Release
IntegraGen and Advanced Biological Laboratories sign global licensing agreement for the commercialization of miRpredX test
ÉVRY, France and LUXEMBOURG City, Luxembourg (February 27th, 2019) – IntegraGen, a company specializing in the transformation of data from biological samples into genomic information and diagnostic tools for oncology, and Advanced Biological Laboratories (ABL), a leading integrated diagnostics and medical IT company, announced today the signing of a non-exclusive global licensing agreement enabling ABL to distribute miRpredX™, IntegraGen’s proprietary IVD CE marked diagnostic test that enables clinicians to identify metastatic colorectal cancer patients who have a higher likelihood of response to anti-EGFR therapy.
See the related Press Release
Early access program launched on ABL Life Science products!
ABL launches its Life Science division and starts the distribution of disruptive molecular biology instruments to be used for research purposes –
Join our Early Access Program and get 30% discount on all UltraGene real time PCR systems!
More information
Join the program
ABL Announces Distribution Agreement with ILC – Instrumentos de Laboratório e Científicos Lda in Portugal
Advanced Biological Laboratories (ABL) S.A. and ILC – Instrumentos de Laboratório e Científicos Lda are pleased to announce that they entered into an exclusive distribution agreement of ABL’s Diagnostics line of products, including the DeepChek® Assays and software, in Portugal.
See current channel partners.
ABL and Xi’an Tianlong Science and Technology Co., Ltd announce a distribution agreement conferring to ABL the ability to commercialize innovative real-time amplification (qPCR) instruments and reagents as well as RNA/DNA automated extraction solutions
ABL to launch its Life Science division and start the distribution of disruptive molecular biology instruments to be used for research purposes.
LUXEMBOURG VILLE, Luxembourg, December 27th, 2018 — Advanced Biological Laboratories (ABL) S.A. today announced the official launch of its Life Science division to provide on a worldwide basis, innovative solutions around molecular biology. It is also proud to announce the execution of a distribution agreement with Xi’an Tianlong Science and Technology Co., Ltd to offer real-time amplification (qPCR) instruments and reagents as well as RNA/DNA extraction solutions in Europe, MEA, LATAM and USA including but not limited to the Gentier qPCR machines and Libex extractors.
Link the press release







