Description
The DeepChek®Assay-HCV CORE Genotyping (RUO) is intended to be used for Viral Hepatitis C (HCV) genotyping and subtyping. It combines target-specific PCR reagents with in vitro diagnostic software both compatible with either Sanger or Next Generation Sequencing platforms.
Methodology
DNA Sequencing • Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
More information on the DeepChek® Assays – Click here
More information on the DeepChek® Software – Click here
Characteristics and performances
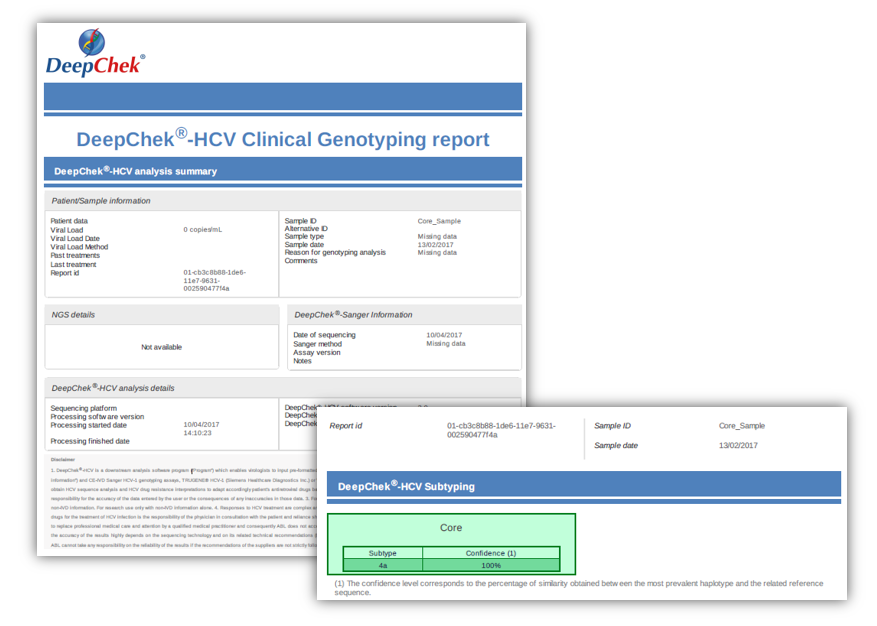
Examples of reports
Ordering information
Downloads
General documentation
-
Protocol
-
Installation check list
-
Q&A
MSDS
-
International