Description
The DeepChek®-HIV INT Genotyping and Drug Resistance Assay V1 (CE-IVD) is intended to be used for HIV-1 genotyping and provides antiretroviral susceptibility information for integrase inhibitors (II). It combines target-specific PCR reagents with in vitro diagnostic software both compatible with either Sanger or Next Generation Sequencing platforms.
Methodology
DNA Sequencing • Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Clinical Significance
The emergence of integrase drug resistance mutations has been observed in vitro and in patients experiencing virologic failure on raltegravir in clinical trials. Twenty three percent of patients receiving raltegravir in a clinical trial experienced virologic failure at 48 weeks and genotypic analysis detected raltegravir associated resistance mutations in 68% of virologic failures. This assay amplifies and sequences the HIV-1 integrase gene and reports mutations at positions associated with integrase inhibitor drug resistance.
More information on the DeepChek® Assays – Click here
More information on the DeepChek® Software – Click here
Characteristics and performances
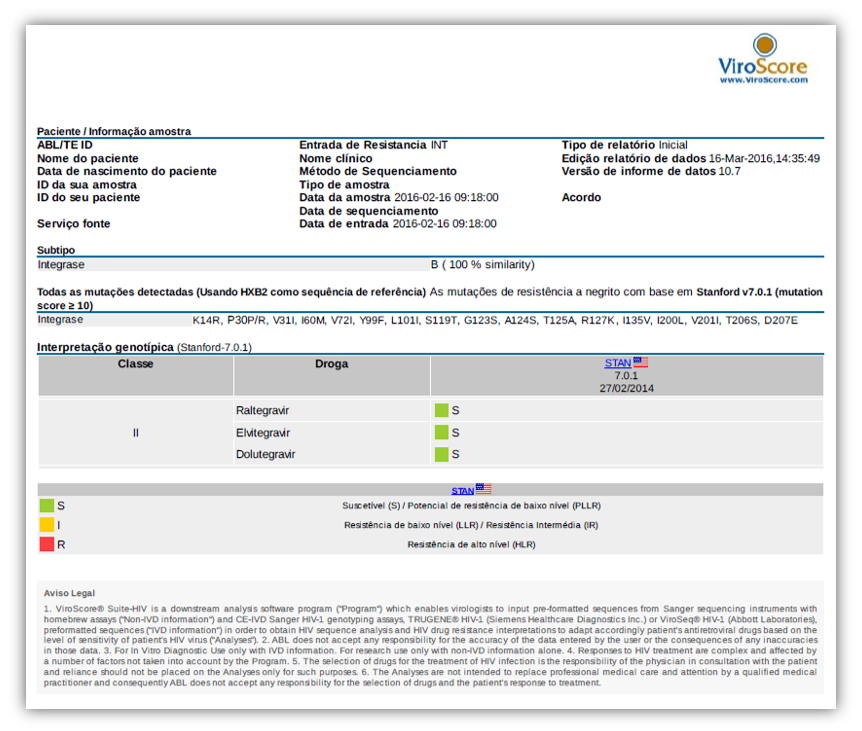
Examples of reports
For SANGER sequencing
For NGS sequencing

Ordering information
General document
-
EC Declaration of Conformity (122A24)
Downloads
General documentation
-
Protocol
-
Installation check list
-
Q&A
MSDS
-
International