Description
The DeepChek® Assay -HIV Assay Protease / Reverse Transcriptase Genotyping and Drug Resistance ![]() is intended to be used for HIV-1 genotyping and provides antiretroviral susceptibility information for protease inhibitors (PI) and reverse transcriptase inhibitors (NRTI, NNRTI). It combines target-specific PCR reagents with in vitro diagnostic software both compatible with either Sanger or Next Generation Sequencing platforms.
is intended to be used for HIV-1 genotyping and provides antiretroviral susceptibility information for protease inhibitors (PI) and reverse transcriptase inhibitors (NRTI, NNRTI). It combines target-specific PCR reagents with in vitro diagnostic software both compatible with either Sanger or Next Generation Sequencing platforms.
Methodology
DNA Sequencing • Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
More information on the DeepChek® Assays – Click here
More information on the DeepChek® Software – Click here
This test is not available for sale in the U.S. – CE marking only valid for EEA and territories recognizing it – Otherwise For Research Use Only (RUO). Not for use in diagnostic procedures. No claim or representation is intended to provide information for the diagnosis, prevention, or treatment of disease. See product REF 101B24.
Characteristics and performances
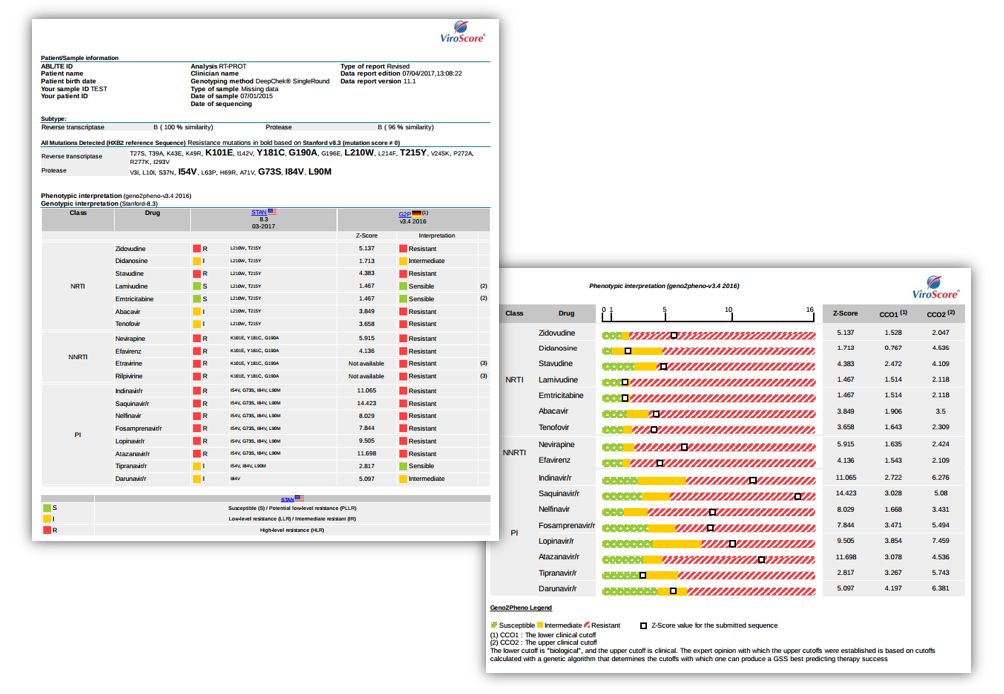
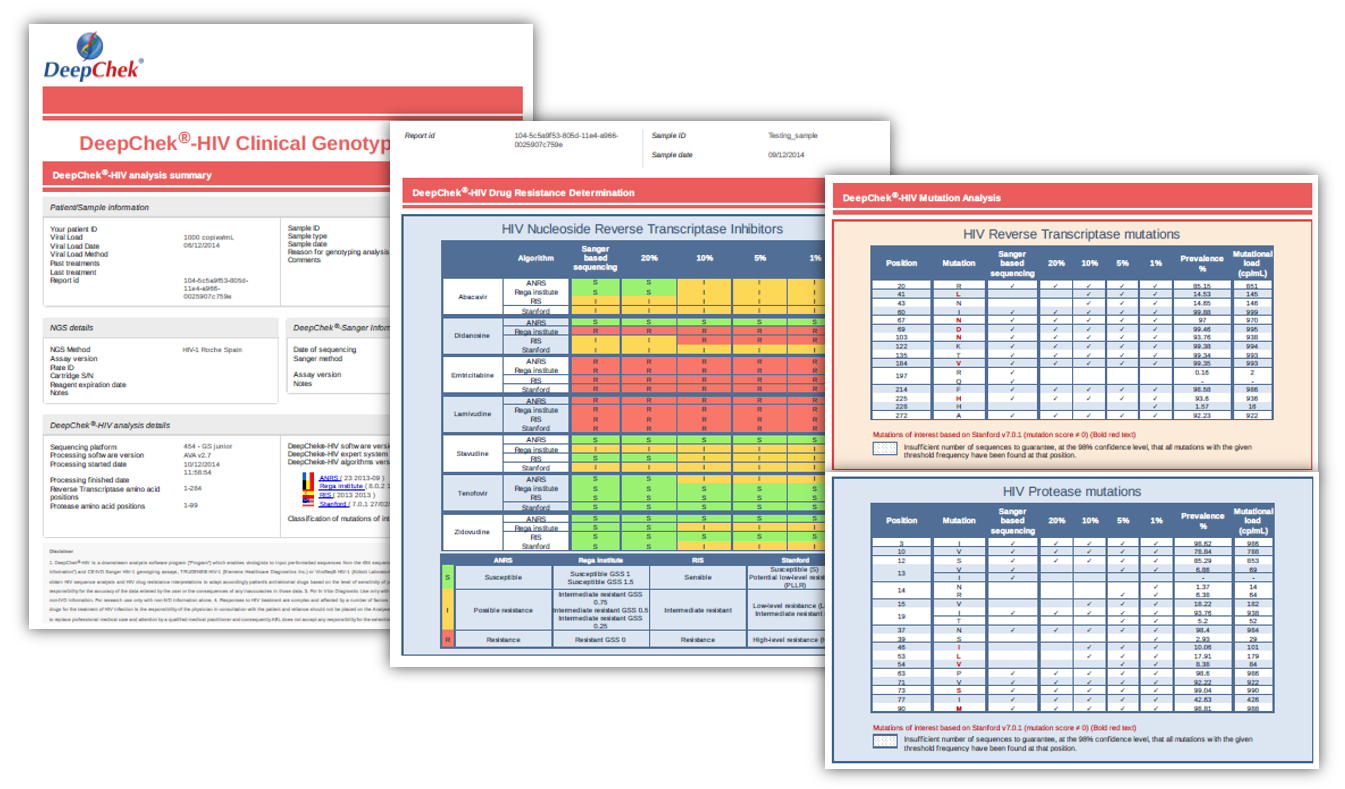
Examples of reports
For SANGER sequencing
For NGS sequencing
Ordering information
Downloads
General documentation
-
Protocol
-
Installation check list
-
EC Declaration of Conformity (121A24)
-
Q&A
MSDS
-
International